| Citation: |
Zhongti Sun, Xiwen Chen, Wanjian Yin. Comprehensive first-principles studies on phase stability of copper-based halide perovskite derivatives AlCumXn (A = Rb and Cs; X = Cl, Br, and I)[J]. Journal of Semiconductors, 2020, 41(5): 052201. doi: 10.1088/1674-4926/41/5/052201
****
Z T Sun, X W Chen, W J Yin, Comprehensive first-principles studies on phase stability of copper-based halide perovskite derivatives AlCumXn (A = Rb and Cs; X = Cl, Br, and I)[J]. J. Semicond., 2020, 41(5): 052201. doi: 10.1088/1674-4926/41/5/052201.
|
Comprehensive first-principles studies on phase stability of copper-based halide perovskite derivatives AlCumXn (A = Rb and Cs; X = Cl, Br, and I)
DOI: 10.1088/1674-4926/41/5/052201
More Information
-
Abstract
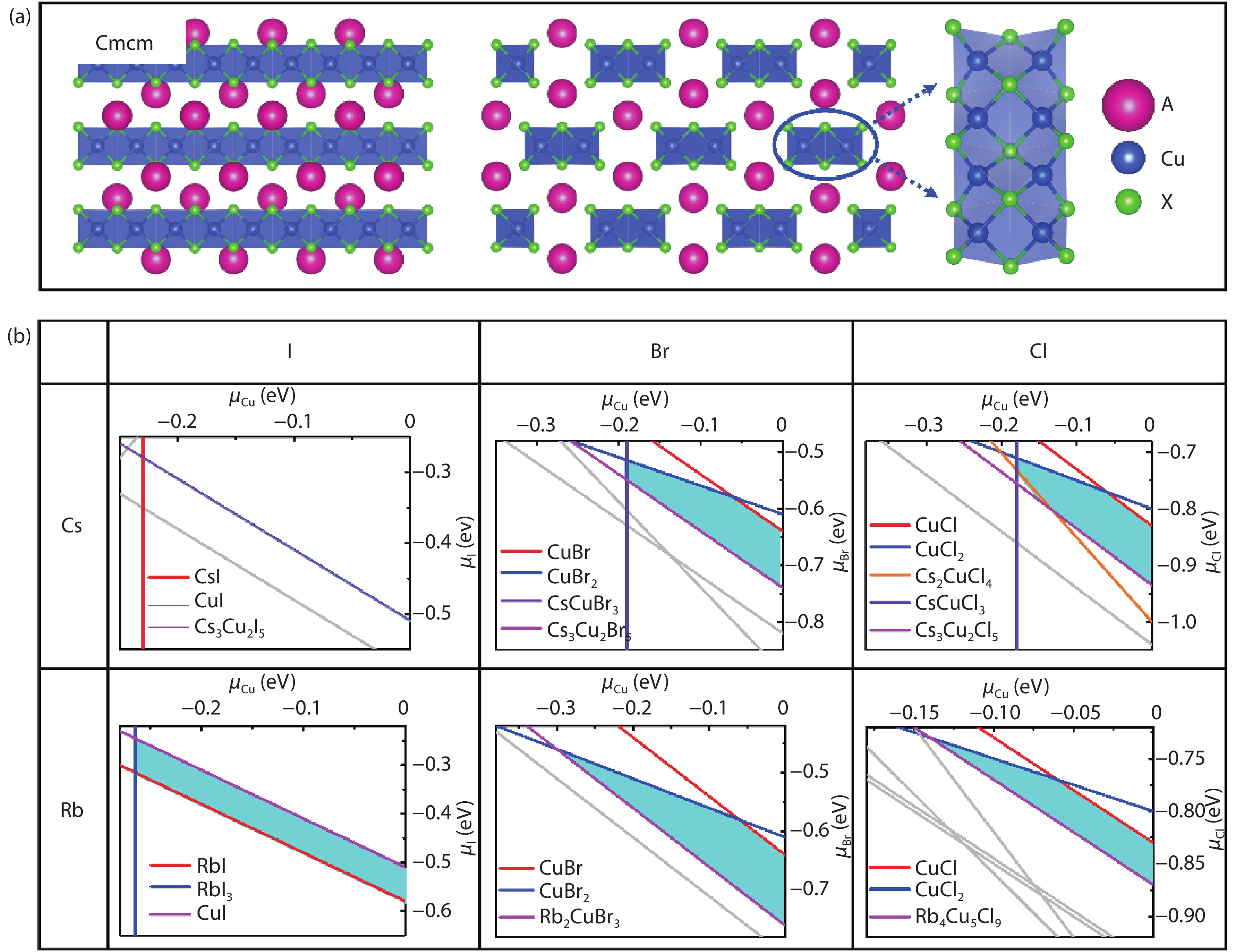
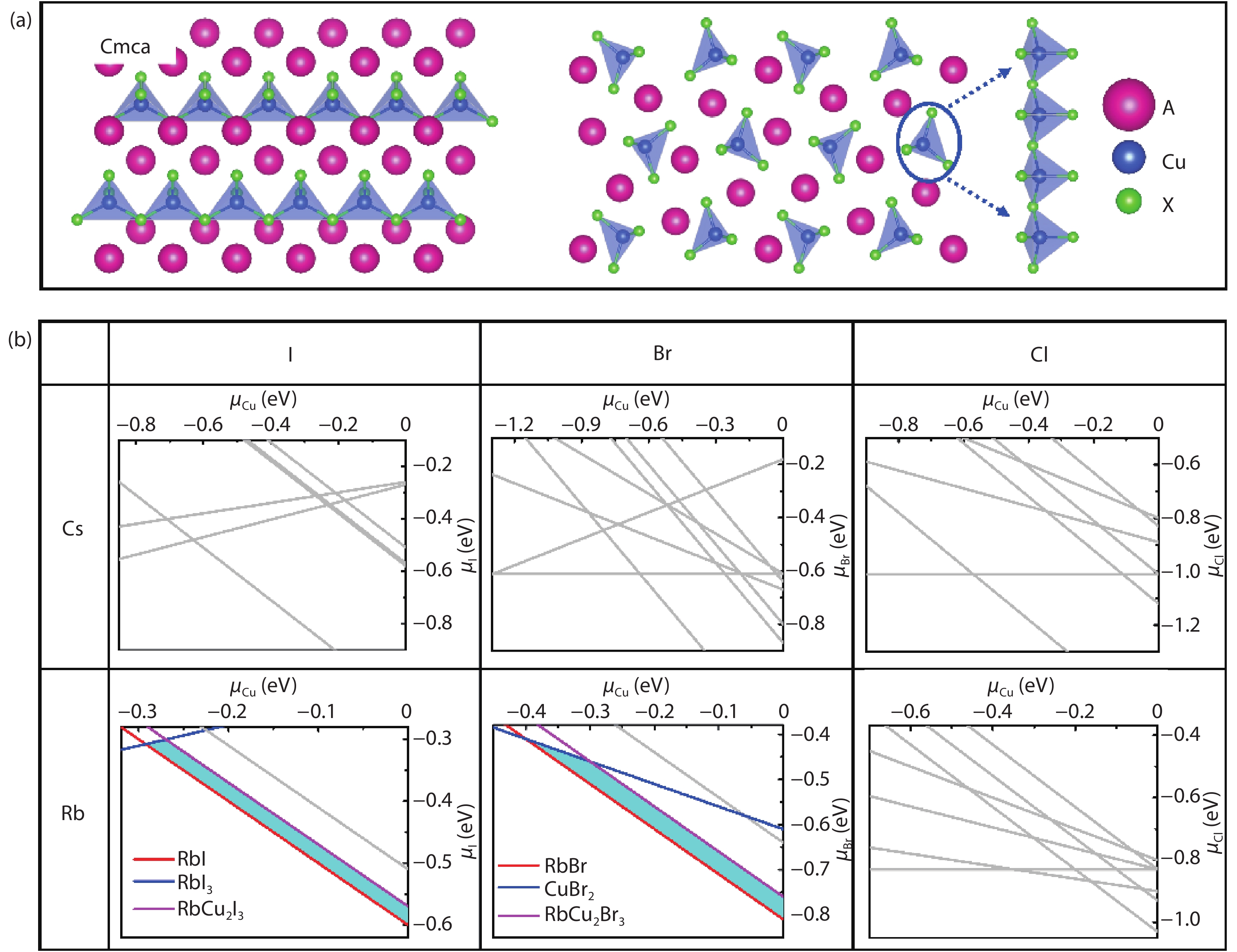
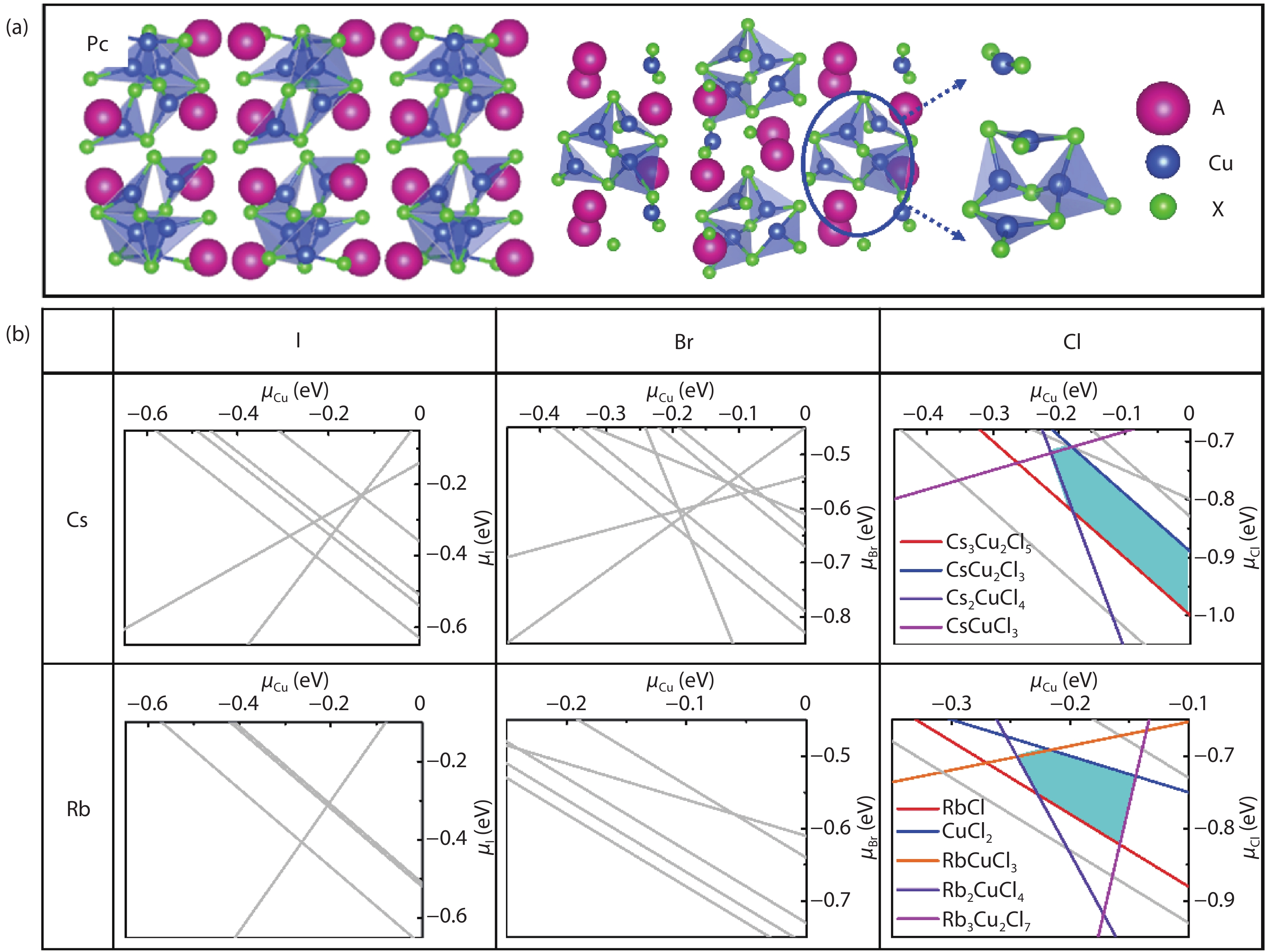
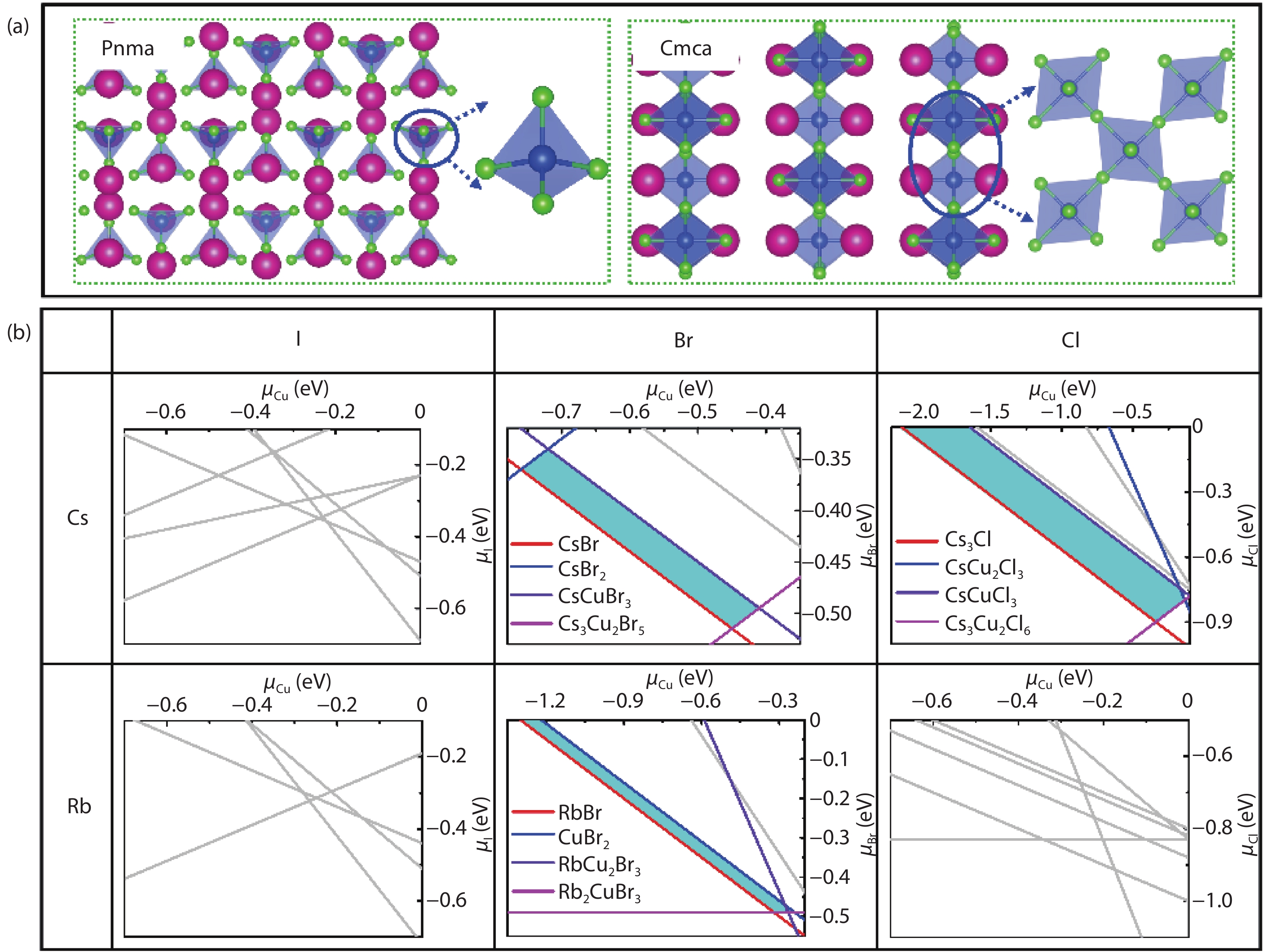
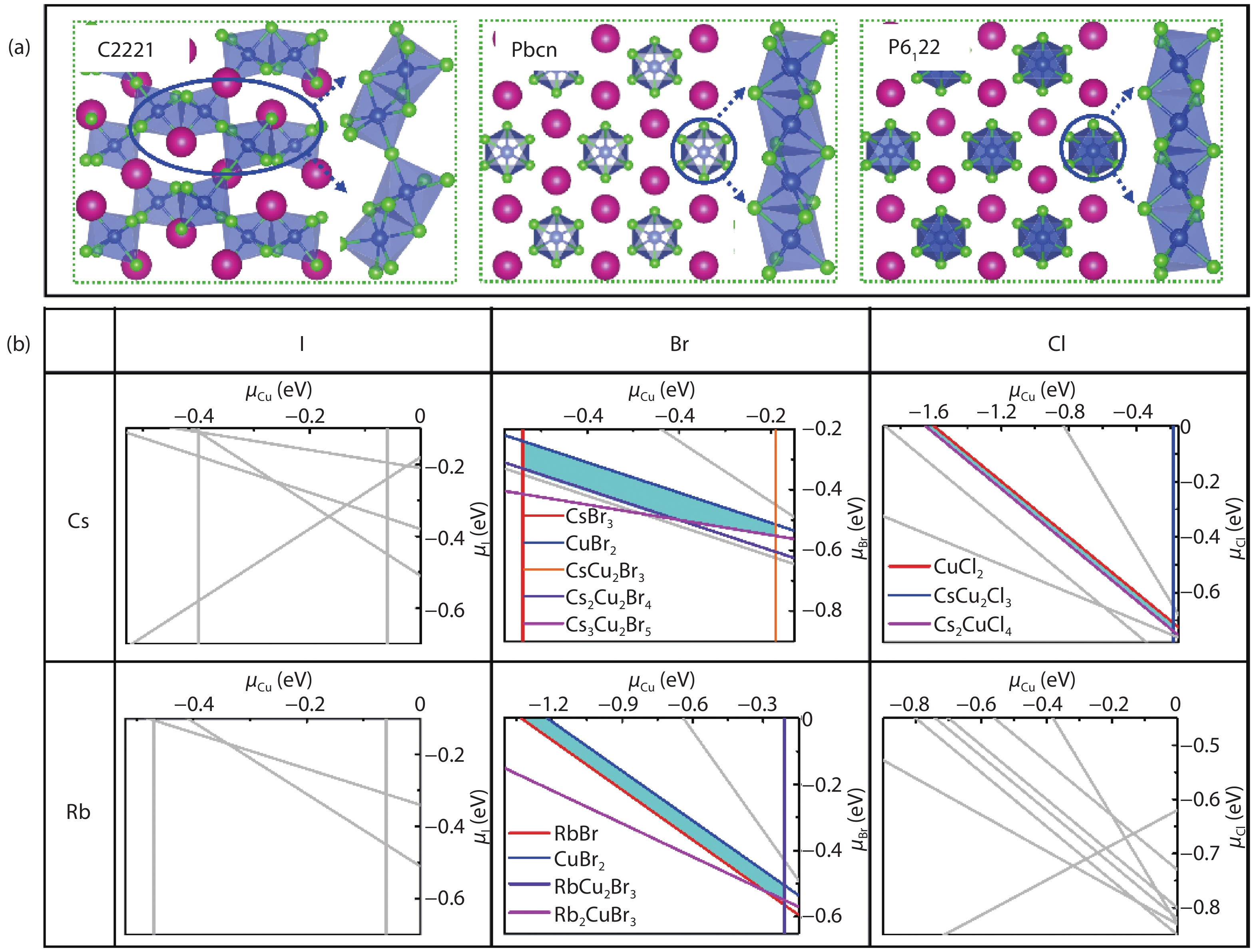
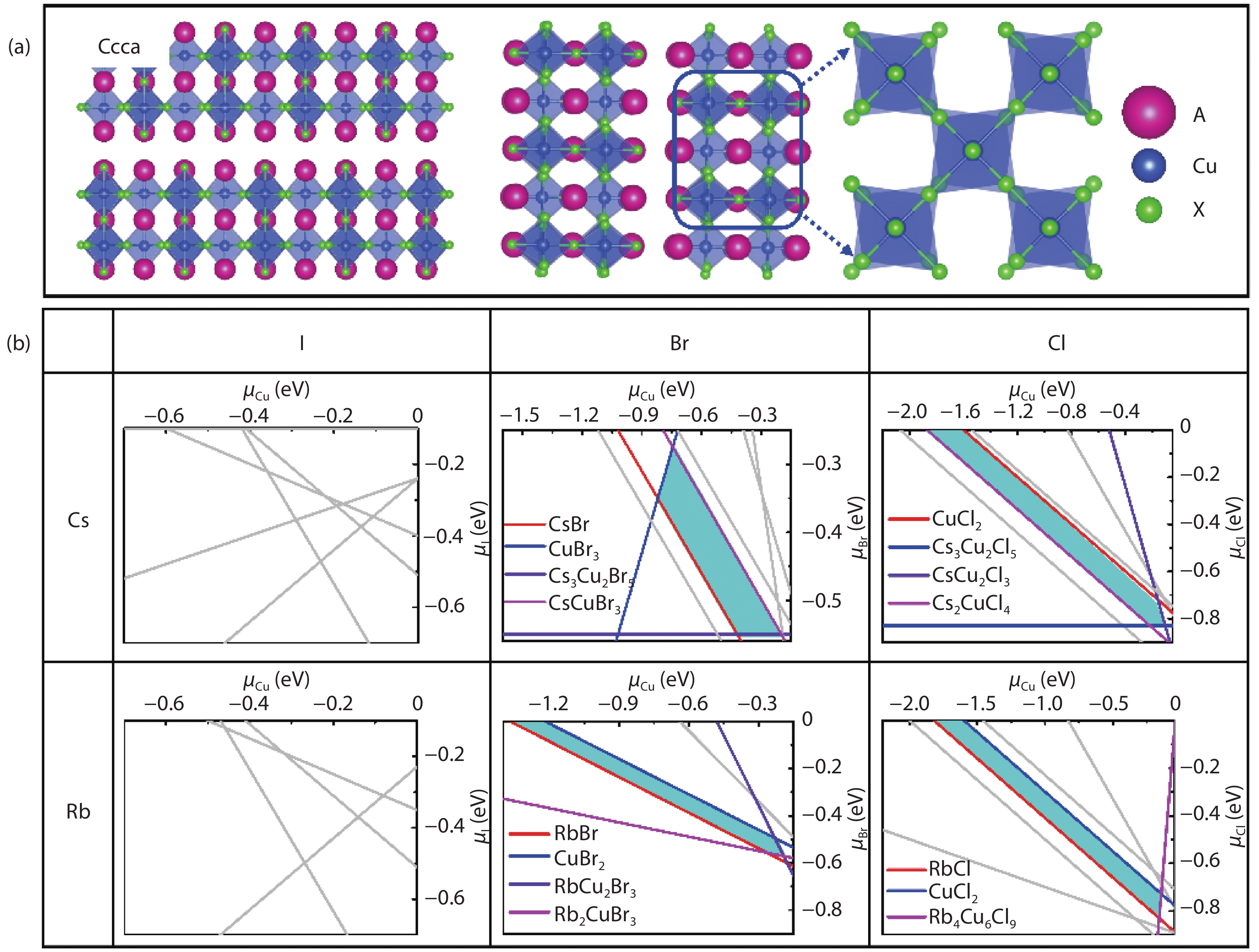
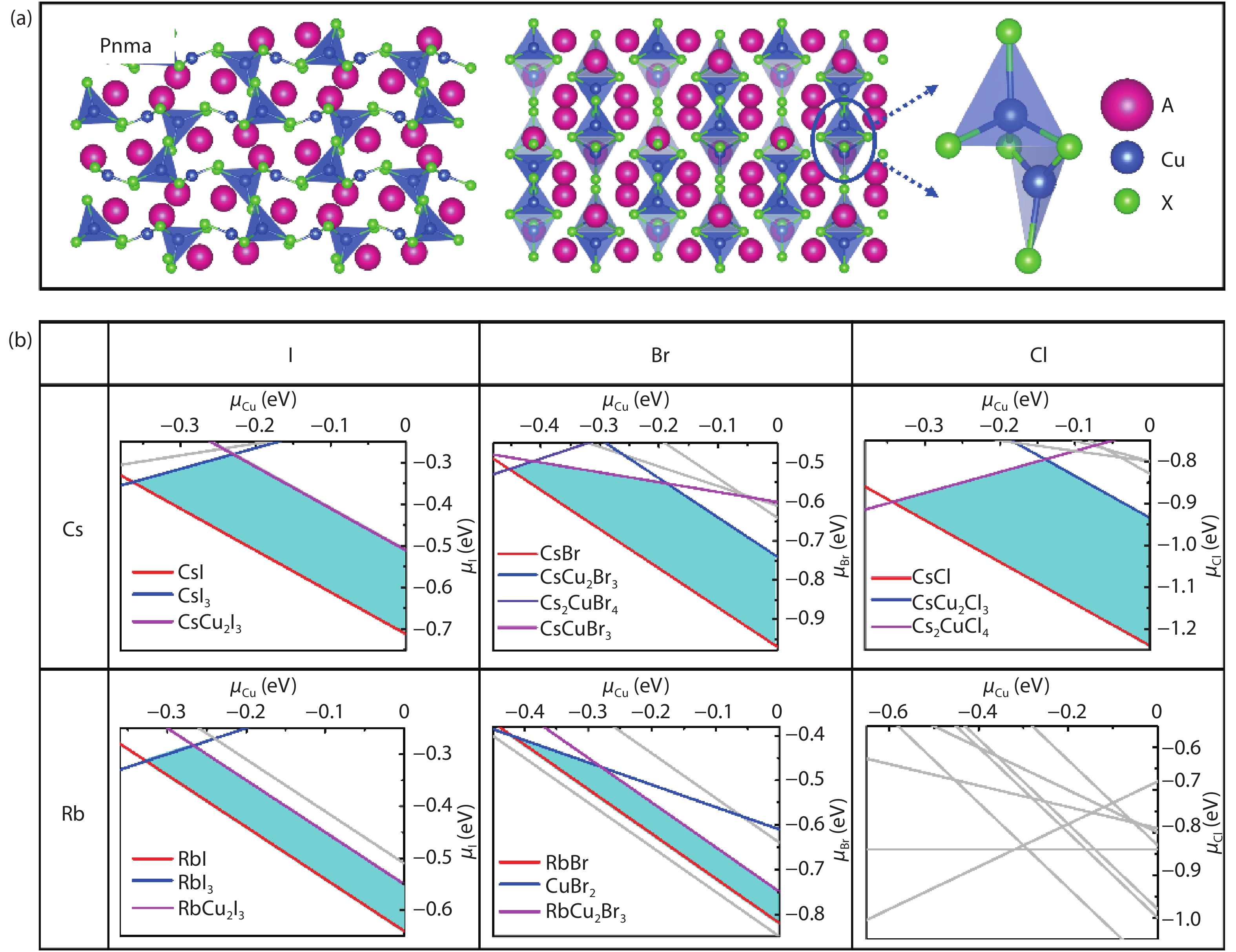
Recently, inorganic copper-based halide perovskites and their derivatives (CHPs) with chemical formulas AlCumXn (A = Rb and Cs; X = Cl, Br and I; l, m, and n are integers.), have received increasing attention in the photoluminescence field, due to their lead-free, cost-effective, earth-abundant and low electronic dimensionality. Ascribed to flexible valence charge of Cu (Cu1+ and Cu2+) and complex competing phases, the crystal structures and phase stabilities of CHPs are complicated and ambiguous, which limits their experimental applications. Via comprehensive first-principles calculations, we have investigated thermodynamic stabilities of possible crystal phases for AlCumXn by considering all the possible secondary phases existing in inorganic crystal structure database (ICSD). Our results are in agreement with existing experiments and further predicted the existence of 10 stable CHPs, i.e. Rb3Cu2Br5, Rb3Cu2I5, RbCu2Cl3, Rb2CuI3, Rb2CuBr4, RbCuBr3, Rb3Cu2Br7, Cs3Cu2Br7, Cs3Cu2Cl7 and Cs4Cu5Cl9, which have not yet been reported in experiments. This work provides a phase and compositional map that may guide experiments to synthesize more novel inorganic CHPs with diverse properties for potential functional applications. -
References
[1] Sun Y, Giebink N C, Kanno H, et al. Management of singlet and triplet excitons for efficient white organic light-emitting devices. Nature, 2006, 440(7086), 908 doi: 10.1038/nature04645[2] Luo J, Wang X, Li S, et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature, 2018, 563(7732), 541 doi: 10.1038/s41586-018-0691-0[3] Tan Z K, Moghaddam R S, Lai M L, et al. Bright light-emitting diodes based on organometal halide perovskite. Nat Nanotechnol, 2014, 9(9), 687 doi: 10.1038/nnano.2014.149[4] Yin W J, Shi T, Yan Y. Unique properties of halide perovskites as possible origins of the superior solar cell performance. Adv Mater, 2014, 26(27), 4653 doi: 10.1002/adma.201306281[5] Cho H, Jeong S H, Park M H, et al. Overcoming the electroluminescence efficiency limitations of perovskite light-emitting diodes. Science, 2015, 350(6265), 1222 doi: 10.1126/science.aad1818[6] Li J, Bade S G R, Shan X, et al. Single-layer light-emitting diodes using organometal halide perovskite/poly(ethylene oxide) composite thin films. Adv Mater, 2015, 27(35), 5196 doi: 10.1002/adma.201502490[7] Saidaminov M I, Almutlaq J, Sarmah S, et al. Pure Cs4PbBr6: highly luminescent zero-dimensional perovskite solids. ACS Energy Lett, 2016, 1(4), 840 doi: 10.1021/acsenergylett.6b00396[8] Cha J H, Han J H, Yin W, et al. Photoresponse of CsPbBr3 and Cs4PbBr6 perovskite single crystals. J Phys Chem Lett, 2017, 8(3), 565 doi: 10.1021/acs.jpclett.6b02763[9] De Bastiani M, Dursun I, Zhang Y, et al. Inside perovskites: quantum luminescence from bulk Cs4PbBr6 single crystals. Chem Mater, 2017, 29(17), 7108 doi: 10.1021/acs.chemmater.7b02415[10] Cortecchia D, Dewi H A, Yin J, et al. Lead-free MA2CuCl xBr4– x hybrid perovskites. Inorg Chem, 2016, 55(3), 1044 doi: 10.1021/acs.inorgchem.5b01896[11] Yang H, Zhang Y, Pan J, et al. Room-temperature engineering of all-inorganic perovskite nanocrsytals with different dimensionalities. Chem Mater, 2017, 29(21), 8978 doi: 10.1021/acs.chemmater.7b04161[12] Yang J, Zhang P, Wei S H. Band structure engineering of Cs2AgBiBr6 perovskite through order–disordered transition: a first-principle study. J Phys Chem Lett, 2017, 9(1), 31 doi: 10.1021/acs.jpclett.7b02992[13] Elseman A M, Shalan A E, Sajid S, et al. Copper-substituted lead perovskite materials constructed with different halides for working (CH3NH3)2CuX4-based perovskite solar cells from experimental and theoretical view. ACS Appl Mater Interfaces, 2018, 10(14), 11699 doi: 10.1021/acsami.8b00495[14] Jun T, Sim K, Iimura S, et al. Lead-free highly efficient blue-emitting Cs3Cu2I5 with 0D electronic structure. Adv Mater, 2018, 30(43), 1804547 doi: 10.1002/adma.201804547[15] Hull S, Berastegui P. Crystal structures and ionic conductivities of ternary derivatives of the silver and copper monohalides — II: ordered phases within the (AgX) x–(MX)1− x and (CuX)x–(MX)1− x (M = K, Rb and Cs; X = Cl, Br and I) systems. J Solid State Chem, 2004, 177(9), 3156 doi: 10.1016/j.jssc.2004.05.004[16] Xiao Z, Du K, Meng W, et al. Chemical origin of the stability difference between copper(I)- and silver(I)-based halide double perovskites. Angew Chem Int Ed, 2017, 129, 12275 doi: 10.1002/ange.201705113[17] Yang P, Liu G, Liu B, et al. All-inorganic Cs2CuX4 (X = Cl, Br, and Br/I) perovskite quantum dots with blue-green luminescence. Chem Commun, 2018, 54(82), 11638 doi: 10.1039/C8CC07118G[18] Helmholz L, Kruh R F. The crystal structure of cesium chlorocuprate, Cs2CuCl4, and the spectrum of the chlorocuprate ion. J Am Chem Soc, 1952, 74(5), 1176 doi: 10.1021/ja01125a012[19] Aguado F, Rodríguez F, Valiente R, et al. Three-dimensional magnetic ordering in the Rb2CuCl4 layer perovskite—structural correlations. J Phys Condens Matter, 2004, 16(12), 1927 doi: 10.1088/0953-8984/16/12/003[20] Lim A R, Kim S H. Study of the structural phase transitions in RbCuCl3 and CsCuCl3 single crystals with the electric-magnetic-type interactions using a 87Rb and 133Cs nuclear magnetic resonance spectrometer. J Appl Phys, 2007, 101, 083519 doi: 10.1063/1.2719290[21] Kousaka Y, Koyama T, Miyagawa M, et al. Crystal growth of chiral magnetic material in CsCuCl3. J Phys Conf Ser, 2014, 502, 012019 doi: 10.1088/1742-6596/502/1/012019[22] Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B, 1996, 54, 11169 doi: 10.1103/PhysRevB.54.11169[23] Blöchl P E. Projector augmented-wave method. Phys Rev B, 1994, 50, 17953 doi: 10.1103/PhysRevB.50.17953[24] Perdew J P, Burke K, Ernzerh of M. Generalized gradient approximation made simple. Phys Rev Lett, 1996, 77, 3865 doi: 10.1103/physrevlett.77.3865[25] Persson C, Zhao Y J, Lany S, et al. n-type doping of CuInSe2 and CuGaSe2. Phys Rev B, 2005, 72(3), 035211 doi: 10.1103/PhysRevB.72.035211[26] Zhao X G, Yang D, Sun Y, et al. Cu–In halide perovskite solar absorbers. J Am Chem Soc, 2017, 139(19), 6718 doi: 10.1021/jacs.7b02120 -
Proportional views





 DownLoad:
DownLoad: