| Citation: |
Xiyue Dong, Dingqin Hu, Pengyu Chen, Xuexin Dai, Chao Hu, Zeyun Xiao, Shirong Lu. Small molecule donors with different conjugated π linking bridges: Synthesis and photovoltaic properties[J]. Journal of Semiconductors, 2020, 41(12): 122201. doi: 10.1088/1674-4926/41/12/122201
****
X Y Dong, D Q Hu, P Y Chen, X X Dai, C Hu, Z Y Xiao, S R Lu, Small molecule donors with different conjugated π linking bridges: Synthesis and photovoltaic properties[J]. J. Semicond., 2020, 41(12): 122201. doi: 10.1088/1674-4926/41/12/122201.
|
Small molecule donors with different conjugated π linking bridges: Synthesis and photovoltaic properties
DOI: 10.1088/1674-4926/41/12/122201
More Information
-
Abstract
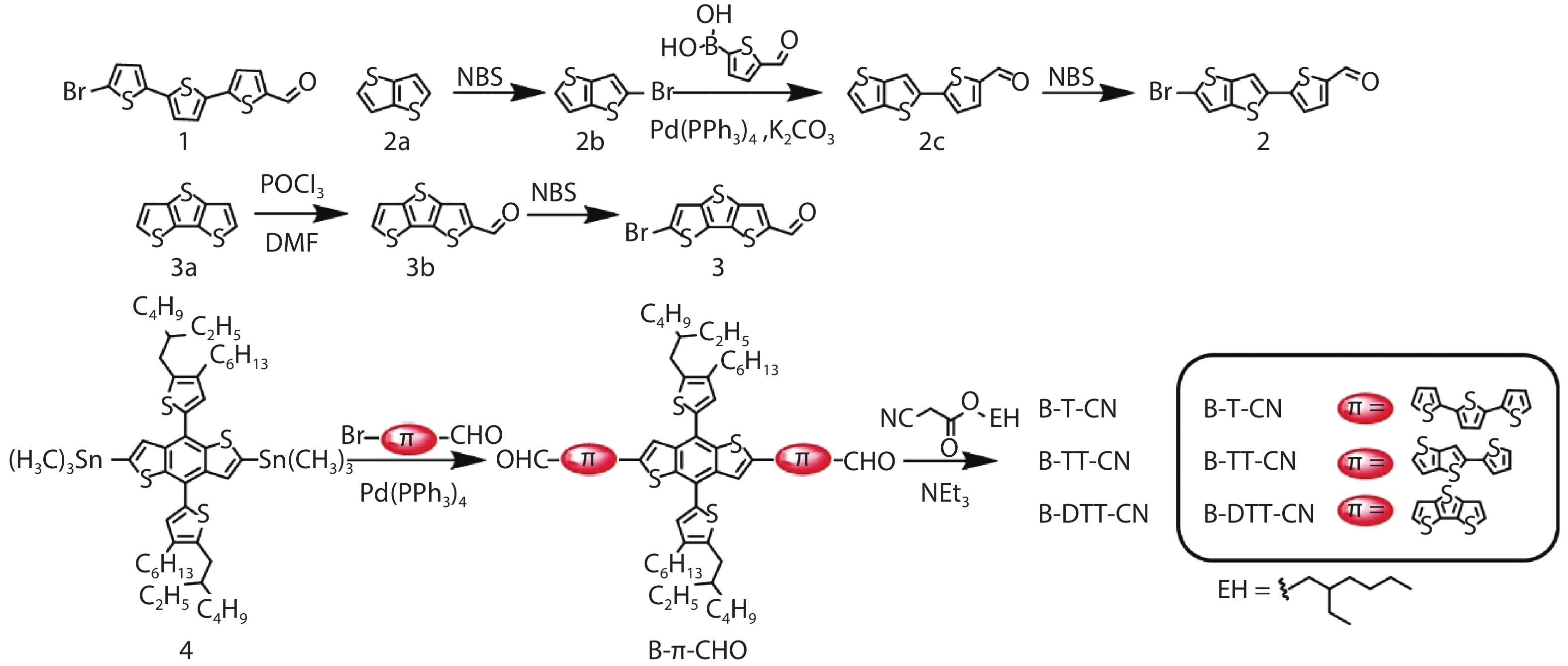
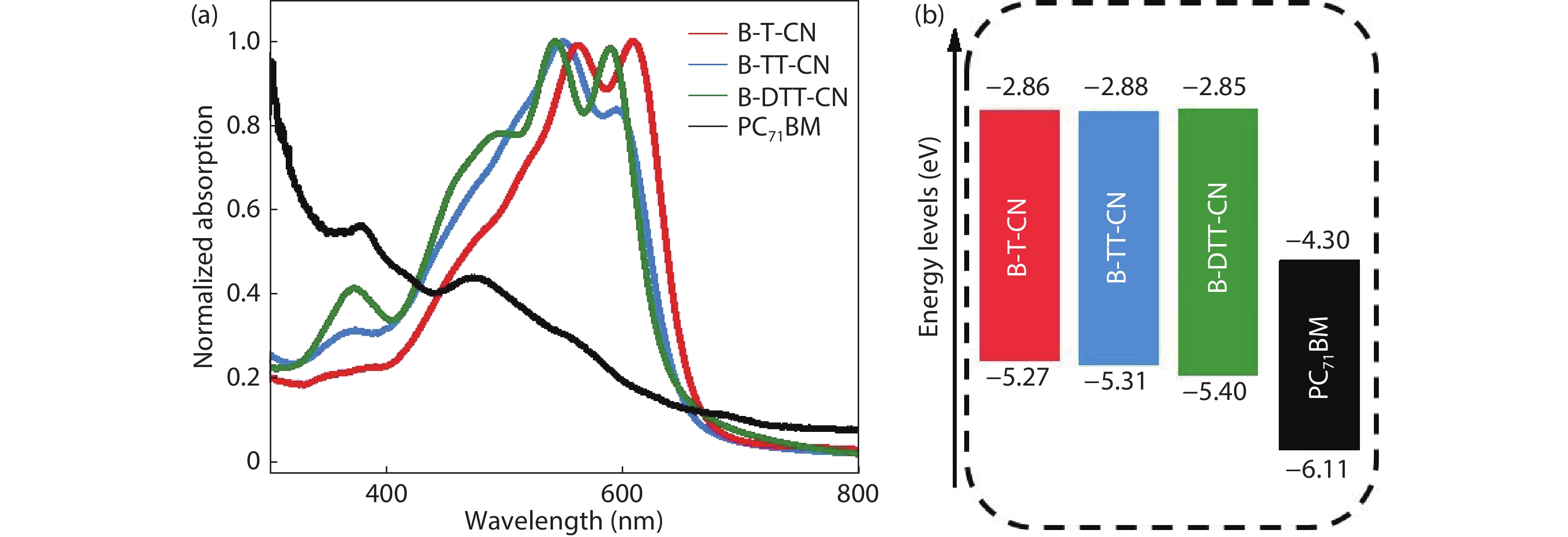
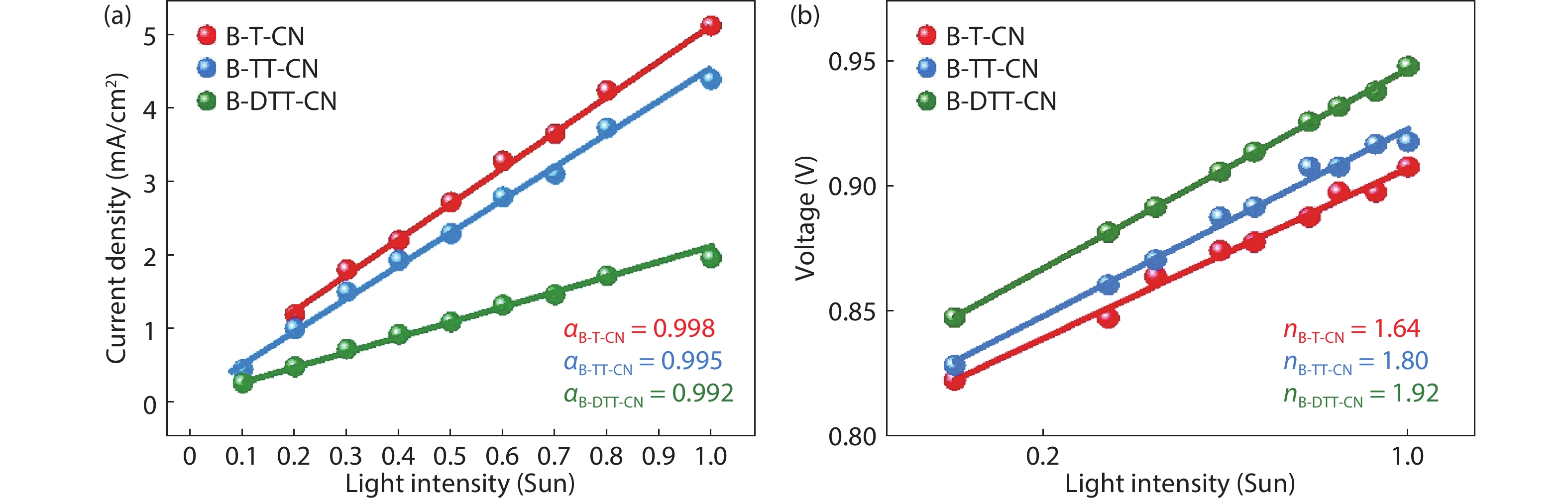
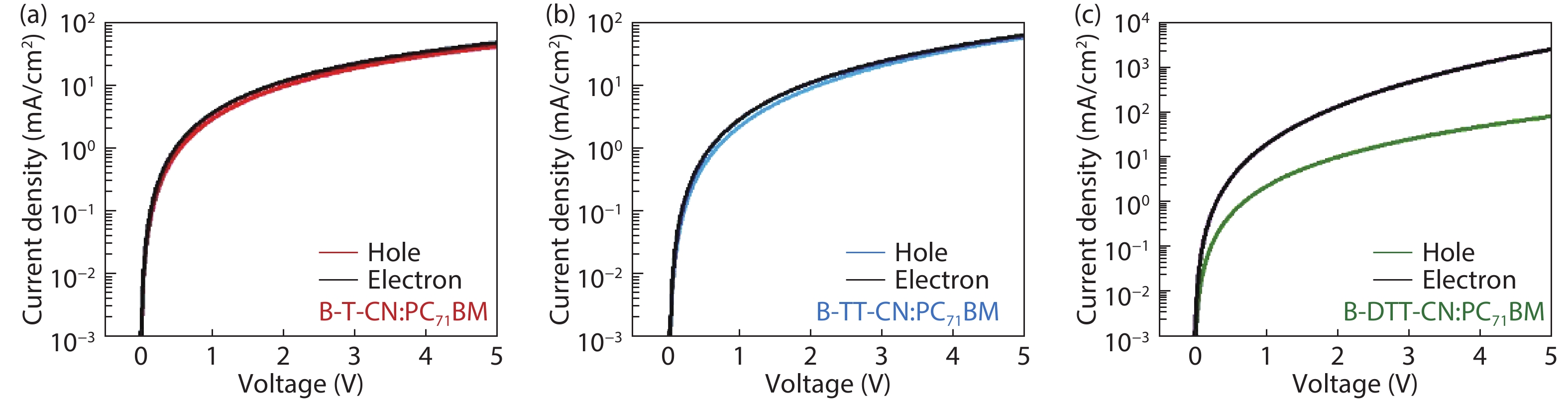
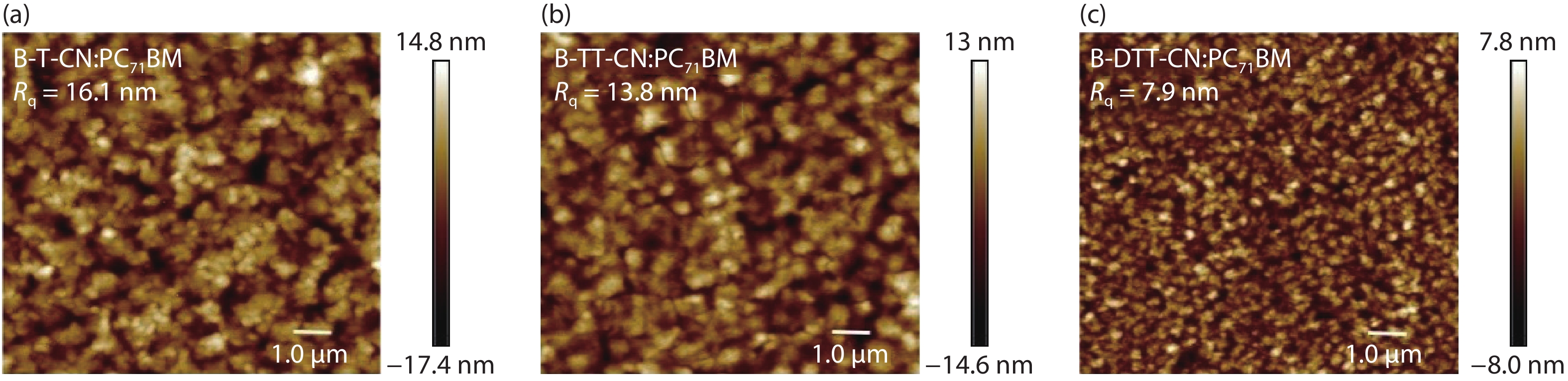
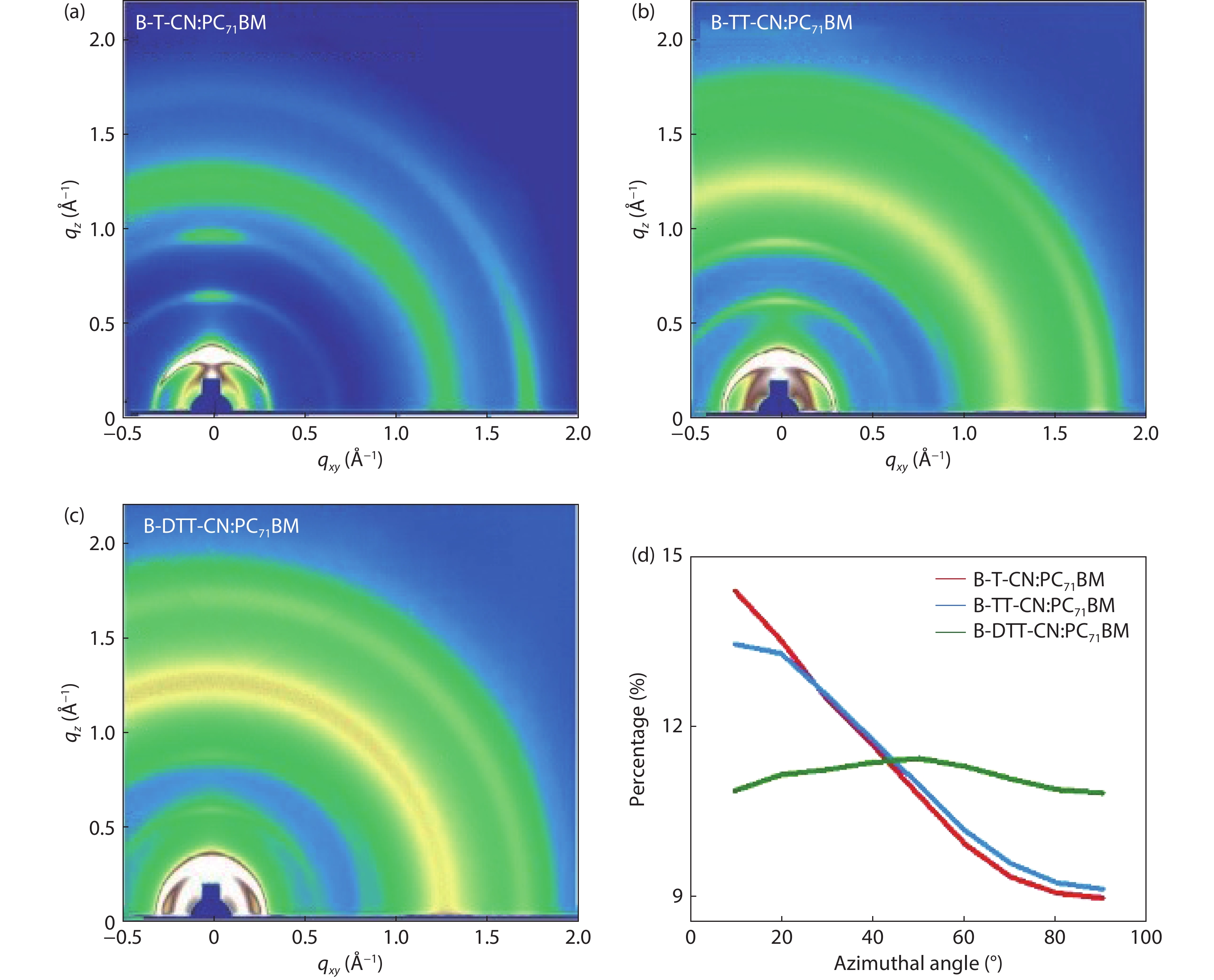
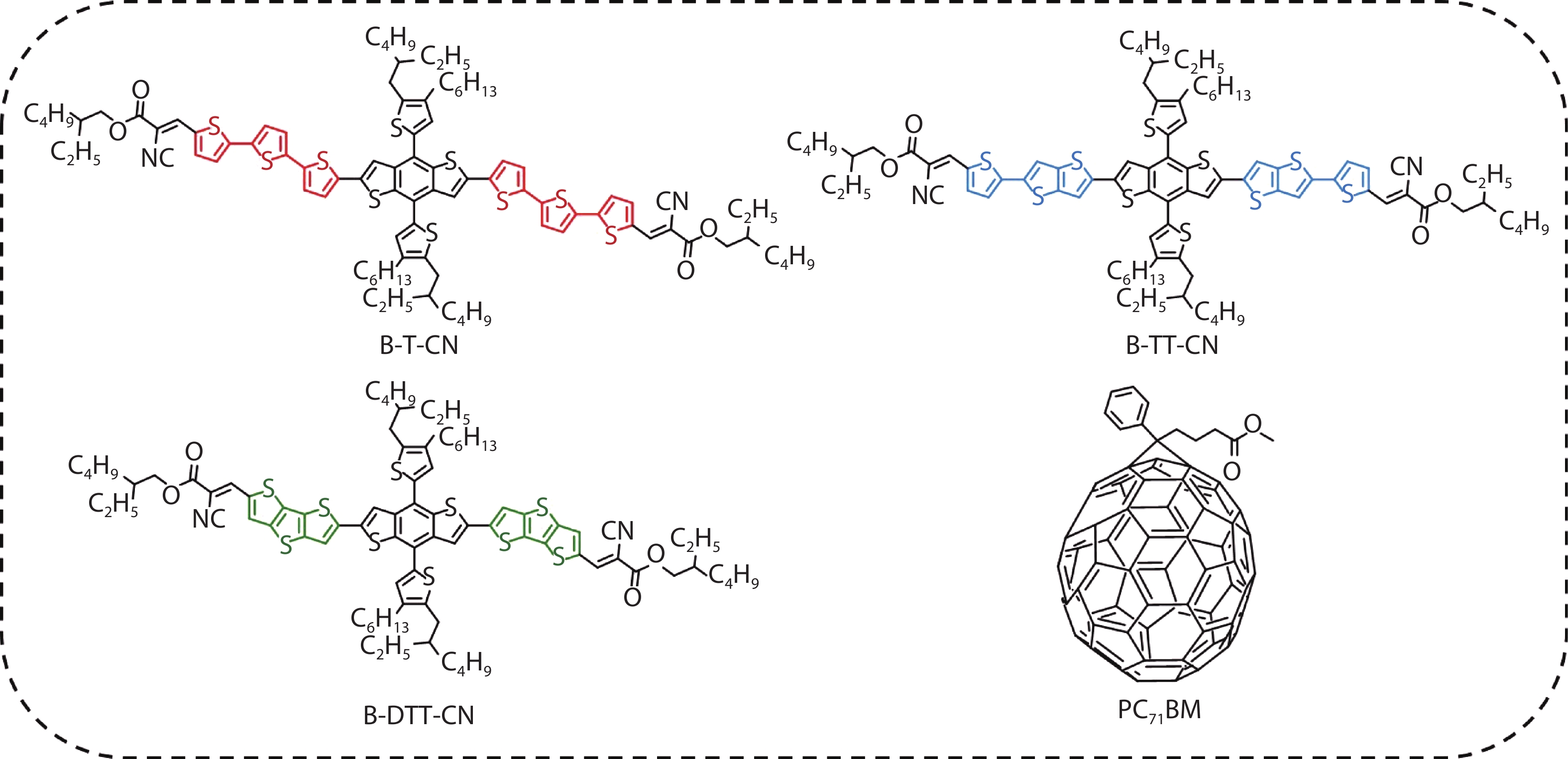
Three small molecule (SM) donors, namely B-T-CN, B-TT-CN and B-DTT-CN, with different π conjugated bridges were synthesized in this research. Interestingly, with the conjugated fused rings of the π linking bridge increasing, the SM HOMO levels exhibit a decline tendency with –5.27 eV for B-T-CN, –5.31 eV for B-TT-CN and –5.40 eV for B-DTT-CN. After blending the SM donors with the fullerene acceptor PC71BM, the all SM organic solar cells (OSCs) achieved high Vocs of 0.90 to 0.96 V. However, the phase separation morphology and molecule stacking are also unexpectedly changed together with the enhancement of conjugated degree of π bridges, resulting in a lower power conversion efficiency (PCE) for the B-DTT-CN:PC71BM device. Our results demonstrate and provide a useful way to enhance OSC Voc and the morphology needs to be further optimized. -
References
[1] Lu L Y, Zheng T Y, Wu Q H, et al. Recent advances in bulk heterojunction polymer solar cells. Chem Rev, 2015, 115, 12666 doi: 10.1021/acs.chemrev.5b00098[2] Zhao F W, Dai S X, Wu Y, et al. Single-junction binary-blend nonfullerene polymer solar cells with 12.1% efficiency. Adv Mater, 2017, 29, 1700144 doi: 10.1002/adma.201700144[3] Lin Y Z, Zhan X W. Oligomer molecules for efficient organic photovoltaics. Acc Chem Res, 2016, 49, 175 doi: 10.1021/acs.accounts.5b00363[4] Hou J, Inganäs O, Friend R H, et al. Organic solar cells based on non-fullerene acceptors. Nat Mater, 2018, 17, 119 doi: 10.1038/nmat5063[5] Liu Q S, Jiang Y F, Jin K, et al. 18% efficiency organic solar cells. Sci Bull, 2020, 65, 272 doi: 10.1016/j.scib.2020.01.001[6] Cui Y, Yao H F, Zhang J Q, et al. Single-junction organic photovoltaic cells with approaching 18% efficiency. Adv Mater, 2020, 32, 1908205 doi: 10.1002/adma.201908205[7] Chen Y S, Wan X J, Long G K. High performance photovoltaic applications using solution-processed small molecules. Acc Chem Res, 2013, 46, 2645 doi: 10.1021/ar400088c[8] Collins S D, Ran N A, Heiber M C, et al. Small is powerful: Recent progress in solution-processed small molecule solar cells. Adv Energy Mater, 2017, 7, 1602242 doi: 10.1002/aenm.201602242[9] Huo Y, Zhang H L, Zhan X W. Nonfullerene all-small-molecule organic solar cells. ACS Energy Lett, 2019, 4, 1241 doi: 10.1021/acsenergylett.9b00528[10] Zhou Z C, Xu S J, Song J N, et al. High-efficiency small-molecule ternary solar cells with a hierarchical morphology enabled by synergizing fullerene and non-fullerene acceptors. Nat Energy, 2018, 3, 952 doi: 10.1038/s41560-018-0234-9[11] Yuan J, Zhang Y Q, Zhou L Y, et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule, 2019, 3, 1140 doi: 10.1016/j.joule.2019.01.004[12] Dong X Y, Yang K, Tang H, et al. Improving molecular planarity by changing alky chain position enables 12.3% efficiency all-small-molecule organic solar cells with enhanced carrier lifetime and reduced recombination. Sol RRL, 2020, 4, 1900326 doi: 10.1002/solr.201900326[13] Yue Q H, Wu H, Zhou Z C, et al. 13.7% efficiency small-molecule solar cells enabled by a combination of material and morphology optimization. Adv Mater, 2019, 31, 1904283 doi: 10.1002/adma.201904283[14] Ge J F, Xie L C, Peng R X, et al. 13.34% efficiency non-fullerene all-small-molecule organic solar cells enabled by modulating the crystallinity of donors via a fluorination strategy. Angew Chem Int Ed, 2020, 59, 2808 doi: 10.1002/anie.201910297[15] Gao J, Ge J F, Peng R X, et al. Over 14% efficiency nonfullerene all-small-molecule organic solar cells enabled by improving the ordering of molecular donors via side-chain engineering. J Mater Chem A, 2020, 8, 7405 doi: 10.1039/D0TA01893G[16] Chen H Y, Hu D Q, Yang Q G, et al. All-small-molecule organic solar cells with an ordered liquid crystalline donor. Joule, 2019, 3, 3034 doi: 10.1016/j.joule.2019.09.009[17] Liu Y S, Wan X J, Wang F, et al. High-performance solar cells using a solution-processed small molecule containing benzodithiophene unit. Adv Mater, 2011, 23, 5387 doi: 10.1002/adma.201102790[18] Kan B, Zhang Q, Li M M, et al. Solution-processed organic solar cells based on dialkylthiol-substituted benzodithiophene unit with efficiency near 10%. J Am Chem Soc, 2014, 136, 15529 doi: 10.1021/ja509703k[19] Zhou J Y, Wan X J, Liu Y S, et al. Small molecules based on benzo[1, 2-b: 4, 5-b’]dithiophene unit for high-performance solution-processed organic solar cells. J Am Chem Soc, 2012, 134, 16345 doi: 10.1021/ja306865z[20] Ni W, Li M M, Wan X J, et al. A high-performance photovoltaic small molecule developed by modifying the chemical structure and optimizing the morphology of the active layer. RSC Adv, 2014, 4, 31977 doi: 10.1039/C4RA04862H[21] Shen S L, Jiang P, He C, et al. Solution-processable organic molecule photovoltaic materials with bithienyl-benzodithiophene central unit and indenedione end groups. Chem Mater, 2013, 25, 2274 doi: 10.1021/cm400782q[22] Sun K, Xiao Z, Lu S, et al. A molecular nematic liquid crystalline material for high-performance organic photovoltaics. Nat Commun, 2015, 6, 6013 doi: 10.1038/ncomms7013[23] Qiu B, Xue L, Yang Y, et al. All-small-molecule nonfullerene organic solar cells with high fill factor and high efficiency over 10%. Chem Mater, 2017, 29, 7543 doi: 10.1021/acs.chemmater.7b02536[24] Bin H J, Yao J, Yang Y K, et al. High-efficiency all-small-molecule organic solar cells based on an organic molecule donor with alkylsilyl-thienyl conjugated side chains. Adv Mater, 2018, 30, 1706361 doi: 10.1002/adma.201706361[25] Wang Y, Liu B, Koh C W, et al. Facile synthesis of polycyclic aromatic hydrocarbon (PAH)-based acceptors with fine-tuned optoelectronic properties: Toward efficient additive-free nonfullerene organic solar cells. Adv Energy Mater, 2019, 9, 1803976 doi: 10.1002/aenm.201803976[26] Wan J H, Xu X P, Zhang G J, et al. Highly efficient halogen-free solvent processed small-molecule organic solar cells enabled by material design and device engineering. Energy Environ Sci, 2017, 10, 1739 doi: 10.1039/C7EE00805H[27] Deng D, Zhang Y J, Zhang J Q, et al. Fluorination-enabled optimal morphology leads to over 11% efficiency for inverted small-molecule organic solar cells. Nat Commun, 2016, 7, 13740 doi: 10.1038/ncomms13740[28] Duan T N, Babics M, Seitkhan A, et al. F-Substituted oligothiophenes serve as nonfullerene acceptors in polymer solar cells with open-circuit voltages >1 V. J Mater Chem A, 2018, 6, 9368 doi: 10.1039/C8TA02781A[29] Liu D X, Kan B, Ke X, et al. Extended conjugation length of nonfullerene acceptors with improved planarity via noncovalent interactions for high-performance organic solar cells. Adv Energy Mater, 2018, 8, 1801618 doi: 10.1002/aenm.201801618[30] Xie Q, Liao X F, Chen L, et al. Random copolymerization realized high efficient polymer solar cells with a record fill factor near 80%. Nano Energy, 2019, 61, 228 doi: 10.1016/j.nanoen.2019.04.048[31] Proctor C M, Kuik M, Nguyen T Q. Charge carrier recombination in organic solar cells. Prog Polym Sci, 2013, 38, 1941 doi: 10.1016/j.progpolymsci.2013.08.008[32] Cowan S R, Roy A, Heeger A J. Recombination in polymer-fullerene bulk heterojunction solar cells. Phys Rev B, 2010, 82, 245207 doi: 10.1103/PhysRevB.82.245207[33] Kirchartz T, Deledalle F, Tuladhar P S, et al. On the differences between dark and light ideality factor in polymer: Fullerene solar cells. J Phys Chem Lett, 2013, 4, 2371 doi: 10.1021/jz4012146[34] Wheeler S, Deledalle F, Tokmoldin N, et al. Influence of surface recombination on charge-carrier kinetics in organic bulk heterojunction solar cells with nickel oxide interlayers. Phys Rev Appl, 2015, 4, 024020 doi: 10.1103/PhysRevApplied.4.024020[35] Liang R Z, Babics M, Savikhin V, et al. Carrier transport and recombination in efficient “all-small-molecule” solar cells with the nonfullerene acceptor IDTBR. Adv Energy Mater, 2018, 8, 1800264 doi: 10.1002/aenm.201800264[36] Zhao F W, Wang C R, Zhan X W. Morphology control in organic solar cells. Adv Energy Mater, 2018, 8, 1703147 doi: 10.1002/aenm.201703147[37] Duan T N, Gao J, Xu T L, et al. Simple organic donors based on halogenated oligothiophenes for all small molecule solar cells with efficiency over 11%. J Mater Chem A, 2020, 8 doi: 10.1039/d0ta00159g[38] Yang L Y, Zhang S Q, He C, et al. Modulating molecular orientation enables efficient non-fullerene small-molecule organic solar cells. Chem Mater, 2018, 30, 30 doi: 10.1021/acs.chemmater.8b00287[39] Vohra V, Kawashima K, Kakara T, et al. Efficient inverted polymer solar cells employing favourable molecular orientation. Nat Photonics, 2015, 9, 403 doi: 10.1038/nphoton.2015.84[40] Jung J, Lee W, Lee C, et al. Controlling molecular orientation of naphthalenediimide-based polymer acceptors for high performance all-polymer solar cells. Adv Energy Mater, 2016, 6, 1600504 doi: 10.1002/aenm.201600504[41] Chen S S, Cho H J, Lee J, et al. Modulating the molecular packing and nanophase blending via a random terpolymerization strategy toward 11% efficiency nonfullerene polymer solar cells. Adv Energy Mater, 2017, 7, 1701125 doi: 10.1002/aenm.201701125 -
Supplements
 20040025suppl.pdf
20040025suppl.pdf

-
Proportional views





 DownLoad:
DownLoad: