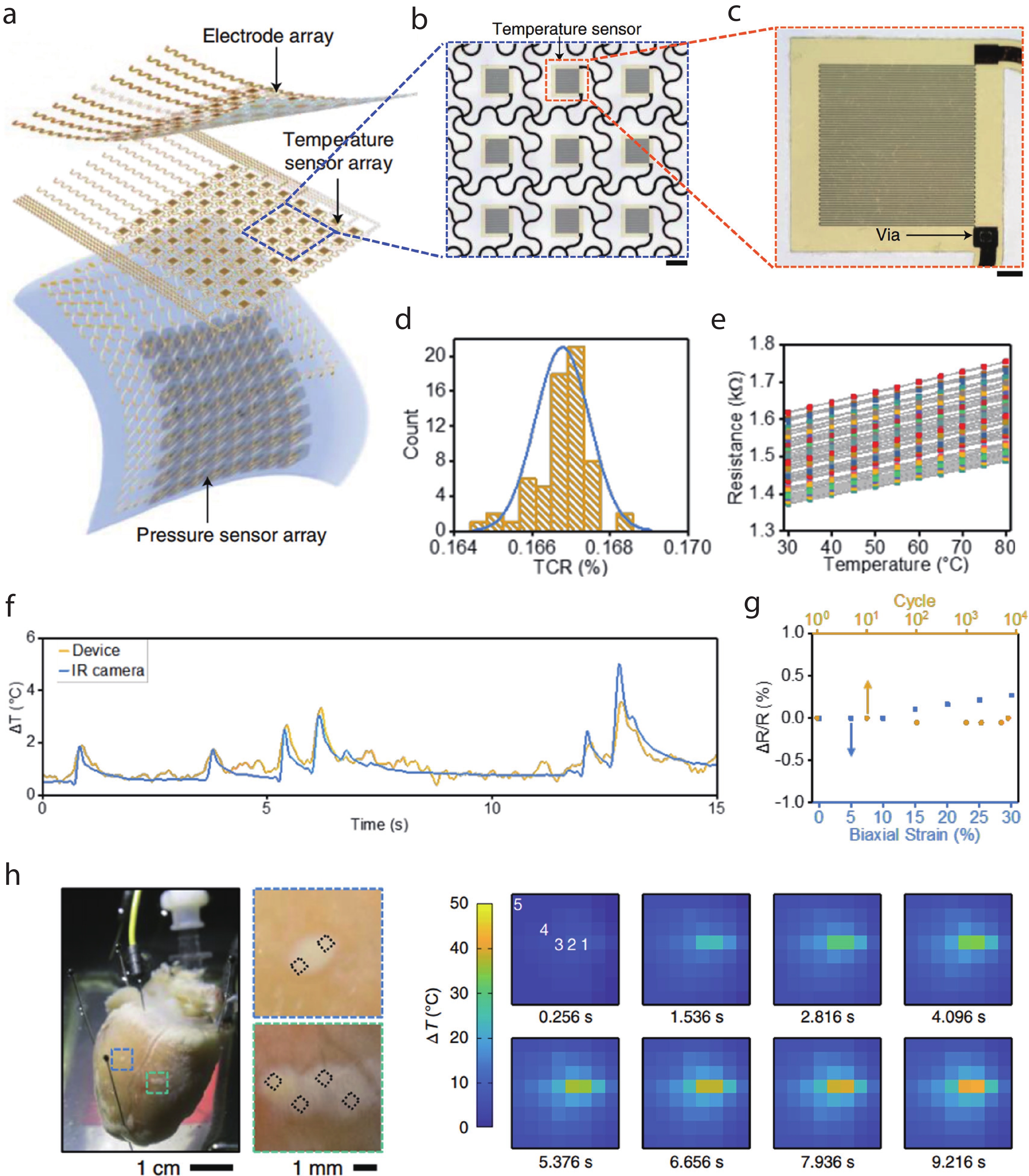
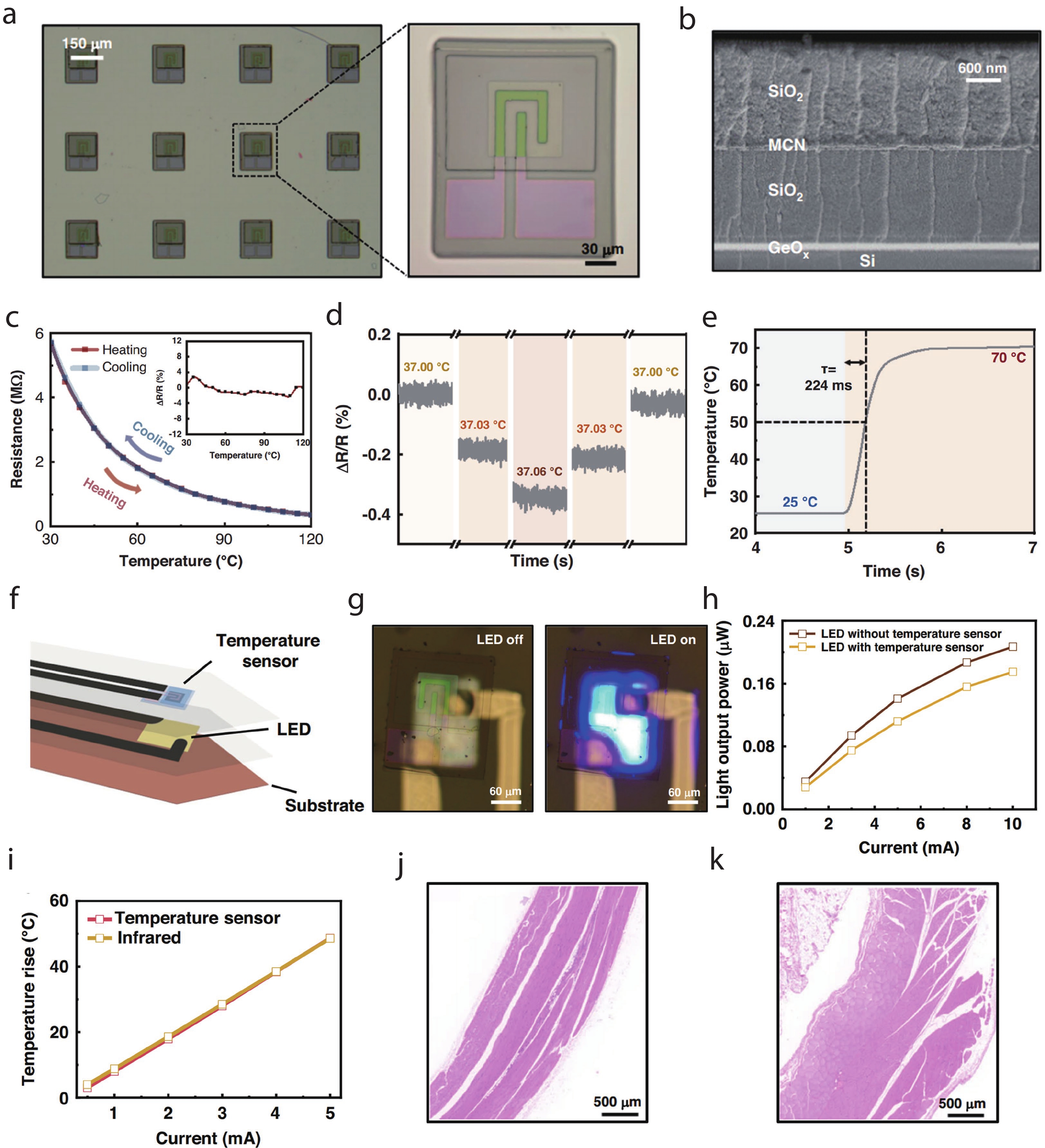
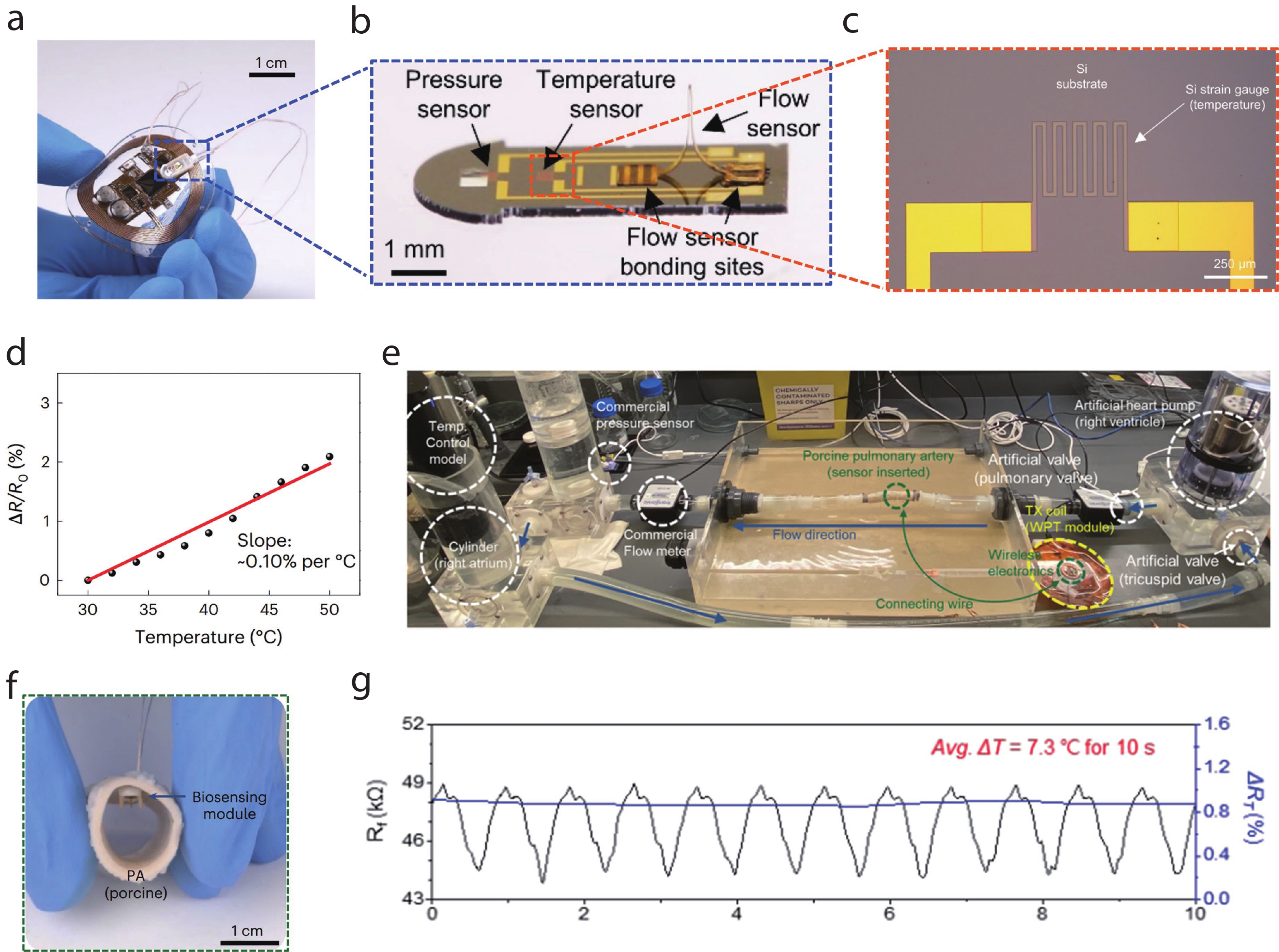
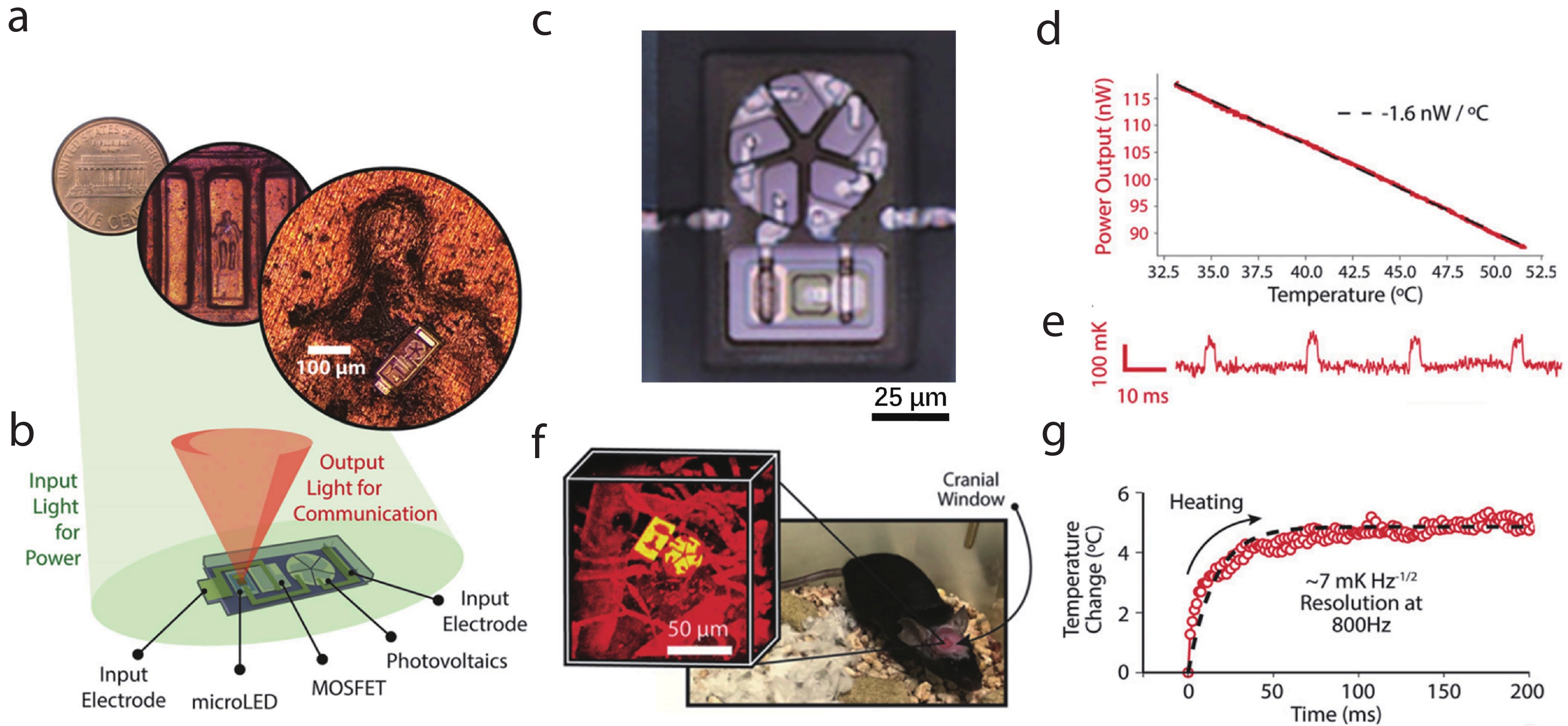
| Citation: |
Zhuofan Yang, Hongcheng Song, He Ding. Advancements in implantable temperature sensors: Materials, mechanisms, and biological applications[J]. Journal of Semiconductors, 2025, 46(1): 011609. doi: 10.1088/1674-4926/24100003
****
Z F Yang, H C Song, and H Ding, Advancements in implantable temperature sensors: Materials, mechanisms, and biological applications[J]. J. Semicond., 2025, 46(1), 011609 doi: 10.1088/1674-4926/24100003
|
Advancements in implantable temperature sensors: Materials, mechanisms, and biological applications
DOI: 10.1088/1674-4926/24100003
CSTR: 32376.14.1674-4926.24100003
More Information-
Abstract
Implantable temperature sensors are revolutionizing physiological monitoring and playing a crucial role in diagnostics, therapeutics, and life sciences research. This review classifies the materials used in these sensors into three categories: metal-based, inorganic semiconductor, and organic semiconductor materials. Metal-based materials are widely used in medical and industrial applications due to their linearity, stability, and reliability. Inorganic semiconductors provide rapid response times and high miniaturization potential, making them promising for biomedical and environmental monitoring. Organic semiconductors offer high sensitivity and ease of processing, enabling the development of flexible and stretchable sensors. This review analyzes recent studies for each material type, covering design principles, performance characteristics, and applications, highlighting key advantages and challenges regarding miniaturization, sensitivity, response time, and biocompatibility. Furthermore, critical performance parameters of implantable temperature sensors based on different material types are summarized, providing valuable references for future sensor design and optimization. The future development of implantable temperature sensors is discussed, focusing on improving biocompatibility, long-term stability, and multifunctional integration. These advancements are expected to expand the application potential of implantable sensors in telemedicine and dynamic physiological monitoring. -
References
[1] Koydemir H C, Ozcan A. Wearable and implantable sensors for biomedical applications. Annual Rev Anal Chem, 2018, 11, 127 doi: 10.1146/annurev-anchem-061417-125956[2] Pang C, Lee C, Suh K Y. Recent advances in flexible sensors for wearable and implantable devices. J Appl Polym Sci, 2013, 130, 1429 doi: 10.1002/app.39461[3] Kiyatkin E A, Brown P L, Wise R A. Brain temperature fluctuation: A reflection of functional neural activation. Eur J Neurosci, 2002, 16, 164 doi: 10.1046/j.1460-9568.2002.02066.x[4] Sim D, Brothers M C, Slocik J M, et al. Biomarkers and detection platforms for human health and performance monitoring: A review. Adv Sci, 2022, 9, 2104426 doi: 10.1002/advs.202104426[5] Cramer M N, Gagnon D, Laitano O, et al. Human temperature regulation under heat stress in health, disease, and injury. Physiol Rev, 2022, 102, 1907 doi: 10.1152/physrev.00047.2021[6] Niedermann R, Wyss E, Annaheim S, et al. Prediction of human core body temperature using non-invasive measurement methods. Int J Biometeorol, 2014, 58, 7 doi: 10.1007/s00484-013-0687-28、10.1002/adhm.202000790[7] Lahiri B B, Bagavathiappan S, Jayakumar T, et al. Medical applications of infrared thermography: A review. Infrared Phys Technol, 2012, 55, 221 doi: 10.1016/j.infrared.2012.03.007[8] Singh R, Bathaei M J, Istif E, et al. A review of bioresorbable implantable medical devices: Materials, fabrication, and implementation. Adv Healthcare Mater, 2020, 9, 2000790 doi: 10.1002/adhm.202000790[9] Ashammakhi N, Hernandez A L, Unluturk B D, et al. Biodegradable implantable sensors: Materials design, fabrication, and applications. Adv Funct Mater, 2021, 31, 2104149 doi: 10.1002/adfm.202104149[10] Lu D, Yan Y, Avila R, et al. Bioresorbable, wireless, passive sensors as temporary implants for monitoring regional body temperature. Adv Healthcare Mater, 2020, 9, 2000942 doi: 10.1002/adhm.202000942[11] Dakurah M N, Koo C, Choi W, et al. Implantable bladder sensors: A methodological review. Int Neurourol J, 2015, 19, 133 doi: 10.5213/inj.2015.19.3.133[12] Scholten K, Meng E. Materials for microfabricated implantable devices: A review. Lab Chip, 2015, 15, 4256 doi: 10.1039/C5LC00809C[13] Koo J H, Song J K, Yoo S, et al. Unconventional device and material approaches for monolithic biointegration of implantable sensors and wearable electronics. Adv Mater Technol, 2020, 5, 2000407 doi: 10.1002/admt.202000407[14] Yang T, Deng W L, Chu X, et al. Hierarchically microstructure-bioinspired flexible piezoresistive bioelectronics. ACS Nano, 2021, 15, 11555 doi: 10.1021/acsnano.1c01606[15] Qin Y, Mo J L, Liu Y H, et al. Stretchable triboelectric self-powered sweat sensor fabricated from self-healing nanocellulose hydrogels. Adv Funct Mater, 2022, 32, 2201846 doi: 10.1002/adfm.202201846[16] He M, Du W W, Feng Y M, et al. Flexible and stretchable triboelectric nanogenerator fabric for biomechanical energy harvesting and self-powered dual-mode human motion monitoring. Nano Energy, 2021, 86, 106058 doi: 10.1016/j.nanoen.2021.106058[17] Andreu-Perez J, Leff D R, Ip H M D, et al. From wearable sensors to smart implants−toward pervasive and personalized healthcare. IEEE Trans Biomed Eng, 2015, 62, 2750 doi: 10.1109/TBME.2015.2422751[18] Liu Y Q, Yu Q, Yang L, et al. Materials and biomedical applications of implantable electronic devices. Adv Mater Technol, 2023, 8, 2200853 doi: 10.1002/admt.202200853[19] Liu Z J, Tian B, Fan X, et al. A temperature sensor based on flexible substrate with ultra-high sensitivity for low temperature measurement. Sens Actuat A Phys, 2020, 315, 112341 doi: 10.1016/j.sna.2020.112341[20] Mailly F, Giani A, Bonnot R, et al. Anemometer with hot platinum thin film. Sens Actuat A Phys, 2001, 94, 32 doi: 10.1016/S0924-4247(01)00668-9[21] Ali M S, Ali M S, Mallick S, et al. Dual parameter smart sensor for nitrogen and temperature sensing based on defect-engineered 1T-MoS2. Sci Rep, 2024, 14, 21469 doi: 10.1038/s41598-024-72632-4[22] Sun X, Li Z C, Fu W Y, et al. Li/Fe modified Zn0.3Ni0.7O NTC thermistors with adjustable resistivities and temperature sensitivity. J Mater Sci Mater Electron, 2018, 29, 343 doi: 10.1007/s10854-017-7922-2[23] Paladiya C, Kiani A. Nano structured sensing surface: Significance in sensor fabrication. Sens Actuat B Chem, 2018, 268, 494 doi: 10.1016/j.snb.2018.04.085[24] Chatterjee S, Saxena M, Padmanabhan D, et al. Futuristic medical implants using bioresorbable materials and devices. Biosens Bioelectron, 2019, 142, 111489 doi: 10.1016/j.bios.2019.111489[25] Wang J Q, Xie H, Chung T, et al. Neural probes with integrated temperature sensors for monitoring retina and brain implantation and stimulation. IEEE Trans Neural Syst Rehabil Eng, 2017, 25, 1663 doi: 10.1109/TNSRE.2016.2634584[26] Moser Y, Gijs M A M. Miniaturized flexible temperature sensor. J Microelectromech Syst, 2007, 16, 1349 doi: 10.1109/JMEMS.2007.908437[27] Xiao S Y, Che L F, Li X X, et al. A novel fabrication process of MEMS devices on polyimide flexible substrates. Microelectron Eng, 2008, 85, 452 doi: 10.1016/j.mee.2007.08.004[28] Li Q, Zhang L N, Tao X M, et al. Review of flexible temperature sensing networks for wearable physiological monitoring. Adv Healthcare Mater, 2017, 6, 1601371 doi: 10.1002/adhm.201601371[29] Shao Z C, Pala S, Liang Y, et al. Non-contact surface temperature sensing based on a single bimorph pMUTS array. 2020 IEEE 33rd International Conference on Micro Electro Mechanical Systems (MEMS), 2020, 861 doi: 10.1109/MEMS46641.2020.9056314[30] Han M D, Chen L, Aras K, et al. Catheter-integrated soft multilayer electronic arrays for multiplexed sensing and actuation during cardiac surgery. Nat Biomed Eng, 2020, 4, 997 doi: 10.1038/s41551-020-00604-w[31] Kim D H, Lu N S, Ghaffari R, et al. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. Nat Mater, 2011, 10, 316 doi: 10.1038/nmat2971[32] Lee S P, Klinker L E, Ptaszek L, et al. Catheter-based systems with integrated stretchable sensors and conductors in cardiac electrophysiology. Proc IEEE, 2015, 103, 682 doi: 10.1109/JPROC.2015.2401596[33] Kim Y, Parada G A, Liu S D, et al. Ferromagnetic soft continuum robots. Sci Robot, 2019, 4, eaax7329 doi: 10.1126/scirobotics.aax7329[34] Yokota T, Zalar P, Kaltenbrunner M, et al. Ultraflexible organic photonic skin. Sci Adv, 2016, 2, e1501856 doi: 10.1126/sciadv.150185639、10.1038/s41528-017-0003-z[35] Arndt-Jovin D J, Robert-Nicoud M, Kaufman S J, et al. Fluorescence digital imaging microscopy in cell biology. Science, 1985, 230, 247 doi: 10.1126/science.4048934[36] Cho M, Han J K, Suh J, et al. Fully bioresorbable hybrid opto-electronic neural implant system for simultaneous electrophysiological recording and optogenetic stimulation. Nat Commun, 2024, 15, 2000 doi: 10.1038/s41467-024-45803-0[37] Jiang N, Chang X H, Hu D W, et al. Flexible, transparent, and antibacterial ionogels toward highly sensitive strain and temperature sensors. Chem Eng J, 2021, 424, 130418 doi: 10.1016/j.cej.2021.130418[38] Cui Y Y, Sun M W, Liu C B, et al. All-inorganic ultrathin high-sensitivity transparent temperature sensor based on a Mn–Co–Ni–O nanofilm. Microsyst Nanoeng, 2024, 10, 70 doi: 10.1038/s41378-024-00706-4[39] Yu K J, Yan Z, Han M D, et al. Inorganic semiconducting materials for flexible and stretchable electronics. NPJ Flex Electron, 2017, 1, 4[40] Liu G, Lv Z Y, Batool S, et al. Biocompatible material-based flexible biosensors: From materials design to wearable/implantable devices and integrated sensing systems. Small, 2023, 19, 2207879 doi: 10.1002/smll.202207879[41] Kwon K, Kim J U, Won S M, et al. A battery-less wireless implant for the continuous monitoring of vascular pressure, flow rate and temperature. Nat Biomed Eng, 2023, 7, 1215 doi: 10.1038/s41551-023-01022-4[42] Milani-Nejad N, Janssen P M L. Small and large animal models in cardiac contraction research: Advantages and disadvantages. Pharmacol Ther, 2014, 141, 235 doi: 10.1016/j.pharmthera.2013.10.007[43] Coffey S, Roberts-Thomson R, Brown A, et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol, 2021, 18, 12 doi: 10.1038/s41569-021-00570-z[44] Charthad J, Weber M J, Chang T C, et al. A mm-sized implantable medical device (IMD) with ultrasonic power transfer and a hybrid bi-directional data link. IEEE J Solid State Circuits, 2015, 50, 1741 doi: 10.1109/JSSC.2015.2427336[45] Lee S, Cortese A J, Gandhi A P, et al. A 250 μm × 57 μm microscale opto-electronically transduced electrodes (MOTEs) for neural recording. IEEE Trans Biomed Circuits Syst, 2018, 12, 1256 doi: 10.1109/TBCAS.2018.2876069[46] Cortese A J, Smart C L, Wang T Y, et al. Microscopic sensors using optical wireless integrated circuits. Proc Natl Acad Sci USA, 2020, 117, 9173 doi: 10.1073/pnas.1919677117[47] Xu M, Zou X M, Su Q Q, et al. Ratiometric nanothermometer in vivo based on triplet sensitized upconversion. Nat Commun, 2018, 9, 2698 doi: 10.1038/s41467-018-05160-1[48] Chen G Y, Qiu H L, Prasad P N, et al. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem Rev, 2014, 114, 5161 doi: 10.1021/cr400425h[49] Chernov M V, Gushchin S V, Kuzmin A M, et al. Infrared to visible up-conversion luminescence of SrF2: Ho particles upon excitation of the 5I7 level of Ho3+ ions. J Lumin, 2023, 261, 119942 doi: 10.1016/j.jlumin.2023.119942[50] Lee G H, Moon H, Kim H, et al. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat Rev Mater, 2020, 5, 149 doi: 10.1038/s41578-019-0167-3[51] Ding H, Lv G Q, Cai X, et al. An Optoelectronic thermometer based on microscale infrared-to-visible conversion devices. Light Sci Appl, 2022, 11, 130 doi: 10.1038/s41377-022-00825-5[52] Shin J, Liu Z H, Bai W B, et al. Bioresorbable optical sensor systems for monitoring of intracranial pressure and temperature. Sci Adv, 2019, 5, eaaw1899 doi: 10.1126/sciadv.aaw1899[53] Zhu C X, Wu H C, Nyikayaramba G, et al. Intrinsically stretchable temperature sensor based on organic thin-film transistors. IEEE Electron Device Lett, 2019, 40, 1630 doi: 10.1109/LED.2019.2933838[54] Someya T, Bao Z N, Malliaras G G. The rise of plastic bioelectronics. Nature, 2016, 540, 379 doi: 10.1038/nature21004[55] Yokota T, Inoue Y, Terakawa Y, et al. Ultraflexible, large-area, physiological temperature sensors for multipoint measurements. Proc Natl Acad Sci USA, 2015, 112, 14533 doi: 10.1073/pnas.1515650112[56] Chen Y, Pépin A. Nanofabrication: Conventional and nonconventional methods. Electrophoresis, 2001, 22, 187 doi: 10.1002/1522-2683(200101)22:2<187::AID-ELPS187>3.0.CO;2-0[57] Mittal M, Sardar S, Jana A. Nanofabrication techniques for semiconductor chemical sensors. Handbook of Nanomaterials for Sensing Applications. Amsterdam: Elsevier, 2021, 119 doi: 10.1002/1522-2683(200101)22:2%3C187::AID-ELPS187%3E3.0.CO;2-0[58] Ahmad S. Organic semiconductors for device applications: Current trends and future prospects. J Polym Eng, 2014, 34, 279 doi: 10.1515/polyeng-2013-0267[59] Eslamian M. Inorganic and organic solution-processed thin film devices. Nanomicro Lett, 2017, 9, 3 doi: 10.1007/s40820-016-0106-4[60] Vishwakarma A, Bhise N S, Evangelista M B, et al. Engineering immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol, 2016, 34, 470 doi: 10.1016/j.tibtech.2016.03.009[61] Zhang D H, Chen Q, Shi C, et al. Dealing with the foreign-body response to implanted biomaterials: Strategies and applications of new materials. Adv Funct Mater, 2021, 31, 2007226 doi: 10.1002/adfm.202007226[62] Ding Y, Ma R C, Liu G H, et al. Fabrication of a new hyaluronic acid/gelatin nanocomposite hydrogel coating on titanium-based implants for treating biofilm infection and excessive inflammatory response. ACS Appl Mater Interfaces, 2023, 15, 13783 doi: 10.1021/acsami.2c23320[63] Basova T V, Vikulova E S, Dorovskikh S I, et al. The use of noble metal coatings and nanoparticles for the modification of medical implant materials. Mater Des, 2021, 204, 109672 doi: 10.1016/j.matdes.2021.109672[64] Kros A, Gerritsen M, Sprakel V S I, et al. Silica-based hybrid materials as biocompatible coatings for glucose sensors. Sens Actuat B Chem, 2001, 81, 68 doi: 10.1016/S0925-4005(01)00933-9[65] Chen K, Ren J Y, Chen C Y, et al. Safety and effectiveness evaluation of flexible electronic materials for next generation wearable and implantable medical devices. Nano Today, 2020, 35, 100939 doi: 10.1016/j.nantod.2020.100939 -
Proportional views





 Zhuofan Yang received his BS degree from Beijing Forestry University in 2020. He is now a postgraduate student of Beijing Institute of Technology, under the guidance of Professor He Ding.
Zhuofan Yang received his BS degree from Beijing Forestry University in 2020. He is now a postgraduate student of Beijing Institute of Technology, under the guidance of Professor He Ding. Hongcheng Song received his Ph.D. from Capital Medical University, Beijing, China, in 2012. He pursued advanced studies in urology at Morgan Stanley Children's Hospital of New York and Children's Hospital Los Angeles in 2014. He is currently a chief physician at Beijing Children's Hospital and an Associate Professor at Capital Medical University.
Hongcheng Song received his Ph.D. from Capital Medical University, Beijing, China, in 2012. He pursued advanced studies in urology at Morgan Stanley Children's Hospital of New York and Children's Hospital Los Angeles in 2014. He is currently a chief physician at Beijing Children's Hospital and an Associate Professor at Capital Medical University. He Ding received his Ph.D. from Ecole Centrale de Lyon, France, in 2016 and postdoctoral studies at Tsinghua University, Beijing, China, from 2016 to 2018. He is currently an Associate Professor at the School of Optics and Photonics, Beijing Institute of Technology, Beijing.
He Ding received his Ph.D. from Ecole Centrale de Lyon, France, in 2016 and postdoctoral studies at Tsinghua University, Beijing, China, from 2016 to 2018. He is currently an Associate Professor at the School of Optics and Photonics, Beijing Institute of Technology, Beijing.
 DownLoad:
DownLoad: