| Citation: |
Safa'a M. Hraibat, Rushdi M-L. Kitaneh, Mohammad M. Abu-Samreh, Abdelkarim M. Saleh. AC-electronic and dielectric properties of semiconducting phthalocyanine compounds:a comparative study[J]. Journal of Semiconductors, 2013, 34(11): 112001. doi: 10.1088/1674-4926/34/11/112001
****
S M Hraibat, R M L Kitaneh, M M Abu-Samreh, A M Saleh. AC-electronic and dielectric properties of semiconducting phthalocyanine compounds:a comparative study[J]. J. Semicond., 2013, 34(11): 112001. doi: 10.1088/1674-4926/34/11/112001.
|
AC-electronic and dielectric properties of semiconducting phthalocyanine compounds:a comparative study
DOI: 10.1088/1674-4926/34/11/112001
More Information
-
Abstract
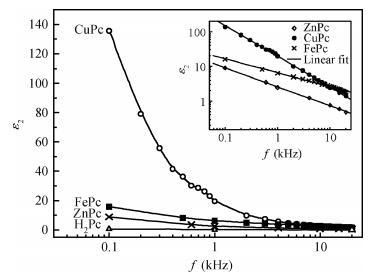
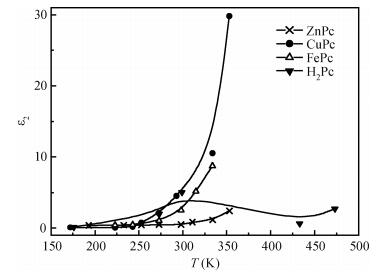
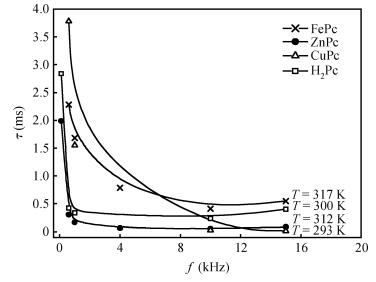
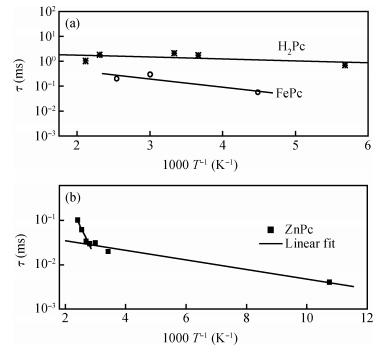
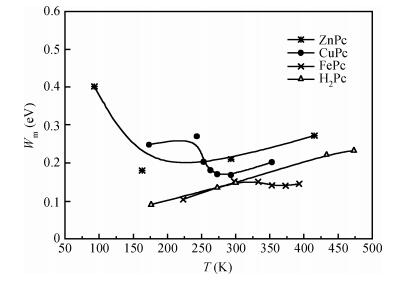
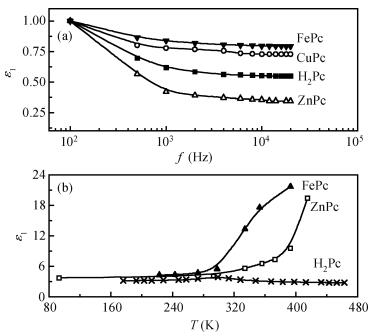
The AC-electronic and dielectric properties of different phthalocyanine films (ZnPc, CuPc, FePc, and H2Pc) were investigated over a wide range of temperature. Both real and imaginary parts of the dielectric constant (ε=ε1-iε2) were found to be influenced by temperature and frequency. Qualitatively the behavior was the same for those compounds; however, the central atom, film thickness, and the electrode type play an important role in the variation of their values.The relaxation time, τ, was strongly frequency-dependent at all temperatures and low frequencies, while a weak dependency is observed at higher frequencies. The relaxation activation energy was derived from the slopes of the fitted lines of ln τ and the reciprocal of the temperature (1/T). The values of the activation energy were accounted for the hopping process at low temperatures, while a thermally activated conduction process was dominant at higher temperatures.The maximum barrier height, Wm, was found to be temperature and frequency dependent for all phthalocyanine compounds. The value Wm depends greatly on the nature of the central atom and electrode material type. The correlated barrier hopping model was found to be the appropriate mechanism to describe the charge carrier's transport in phthalocyanine films. -
References
[1] Wilson A Collins R A. Electrical characteristics of planar phthalocyanine thin film gas sensors. Sensors and Actuators, 1987, 12(4):389 doi: 10.1016/0250-6874(87)80058-6[2] Collins R A, Ellis M K, Jones T A, Sensitivity of lead phthalocyanine thin films to ammonia and nitrogen dioxide. Chemtronics, 1991, 5:93 doi: 10.1007/s11534-005-0008-4[3] Gould R D, Hassan A K. AC electrical properties of thermally evaporated thin films of copper phthalocyanine. Thin Solid Films, 1993, 223(2):334 doi: 10.1016/0040-6090(93)90541-V[4] Honeybourne C L, Ewen R J. The enhancement of dark d.c. conductivity by gas adsorption on thin films of macrocyclic copper complexes. J Phys Chem Solids, 1983, 44(8):833 doi: 10.1016/0022-3697(83)90017-3[5] Van Ewyk R L, Chadwick A V, Wright J D. Effects of oxygen, nitrogen dioxide and trifluoroborane on photoconductivity of perylene and phthalocyanine single crystals. J Chem Soc, Faraday Trans, 1981, 77(1):73 doi: 10.1039/f19817700073[6] Abu-Hilal A O, Saleh A M, Gould R D. Effect of electrode material on AC electrical conductivity of organic zinc phthalocyanine semiconducting thin films. Mat Chem Phys, 2005, 94(1):165 doi: 10.1016/j.matchemphys.2005.04.027[7] Huo L, Cao L, Li X, et al. Study of ferric oxide nanoparticles-tris-(2, 4-di-t-amylphenoxy)-(8-quinolinolyl) copper phthalocyanine. Thin Solid Films, 2002, 365(1):129 https://ar.scribd.com/document/101017075/13Nparti[8] Ho K C, Tsou Y H. Chemiresistor-type NO gas sensor based on nickel phthalocyanine thin films. Sensors and Actuators B, 2001, 77(1/2):253[9] Rellaa R, Rizzob A, Licciullic A, et al. Tests in controlled atmosphere on new optical gas sensing layers based on TiO2/metal-phthalocyanines hybrid systems. Mater Sci Eng C, 2002, 22(2):439 doi: 10.1016/S0928-4931(02)00193-5[10] Zhilin Z, Xueyin J, Wenquing Z, et al. A white organic light emitting diode with improved stability. J Phys D:Appl Phys, 2001, 34(20):308[11] Henari F Z. Optical switching in organometallic phthalocyanine. J Opt A:Pure Appl Opt, 2001, 3(3):188 doi: 10.1088/1464-4258/3/3/306[12] Fadel M, Kassab K, Fadeel D A. Zinc phthalocyanine-loaded PLGA biodegradable nanoparticles for photodynamic therapy in tumor-bearing mice. Lasers Med Sci, 2010, 25(2):283 doi: 10.1007/s10103-009-0740-x[13] Wihksne K, Newkirk A E. Electrical conductivity of α-and β-phthalocyanine. J Chem Phys, 1961, 34(6):2184 doi: 10.1063/1.1731844[14] Orti E, Brades J L. Electronic structure of metal-free phthalocyanine:a valence effective Hamiltonian theoretical study. J Chem Phys, 1988, 89(2):1009 doi: 10.1063/1.455251[15] Saleh A M, Amar N M, Gould R D. Electrical characterization (a.c. and d.c.).of metal-free phthalocyanine (α -H2Pc) thin films. Current Appl Phys, 2002, 2:455 doi: 10.1016/S1567-1739(02)00156-6[16] Abu-Hilal A O, Gould R D, Abu Taha M I, et al. Electrode material effect on capacitance and loss tangent of the organic ZnPc semiconducting thin films. Int J Mod Phys B, 2007, 21(1):55 doi: 10.1142/S021797920703587X[17] Drechsles U, Pfaff M, Hanack M. Synthesis of novel functionalized zinc phthalocyanines applicable in photodynamic therapy. Europ J Organic Chem, 1999, 1999(12):3441 doi: 10.1002/(ISSN)1099-0690[18] Gao L, Qian X, Zhang L, et al. Tetra-trifluoroethoxyl zinc phthalocyanine:potential photosensitizer for use in the photodynamic therapy of cancer. J Photochemistry and Photobiology, 2001, 65(1):35 doi: 10.1016/S1011-1344(01)00250-0[19] Bowler C J, Gould R D. A sequential masking system for the deposition of multilayer thin film structures. J Vacuum Sci Technol A, 1987, 5(1):114[20] Liao M S, Scheiner S. Electronic structure and bonding in metal phthalocyanine, Metal=Fe, Co, Ni, Cu, Zn, Mg. J Chem Phys, 2001, 114(22):9780 doi: 10.1063/1.1367374[21] Gao W, Kahn A. Electronic structure and current injection in zinc phthalocyanine doped with tetrafluorotetracyanoquin-odimethane:interface versus bulk effects. Org Electron, 2002, 3(2):53 doi: 10.1016/S1566-1199(02)00033-2[22] Shihub S I, Gould R D. Frequency dependence of electrical conduction parameters in evaporated thin films of cobalt phthalocyanine. Thin Solid Films, 1995, 254(1/2):187 doi: 10.1080/00207219208925582[23] Nalwa H S, Vazudevan P. Dielectric properties of cobalt phthalocyanine. J Mat Sci Lett, 1983, 2(1):22 doi: 10.1007/BF00719947[24] Atta A A. AC conductivity and dielectric measurements of bulk magnesium phthalocyanine MgPC. J Alloys Comp, 2009, 480(2):564 doi: 10.1016/j.jallcom.2009.01.124[25] Senthilarasu S, Sathyamoorthy R, Ascencio J A, et al. Dielectric and ac conduction properties of zinc phthalocyanine (ZnPc) thin films. J Appl Phys, 2007, 101(3):034111 doi: 10.1063/1.2435805[26] Kumar S, Husain M, Zulfequar M. Dielectric relaxation in the glassy a-Se-Te-Ga system. Physica B, 2007, 387(1/2):400 http://www.oalib.com/references/7294307[27] Giuntini J C, Zanchetta J V, Jullien D, et al. Temperature dependence of dielectric losses in chalcogenide glasses. J Non-Crystalline Solids, 1981, 45(1):57 doi: 10.1016/0022-3093(81)90089-2[28] Zeyada H M, El-Nahass M M. Electrical properties and dielectric relaxation of thermally evaporated zinc phthalocyanine thin films. Appl Surf Sci, 2008, 254(6):1852 doi: 10.1016/j.apsusc.2007.07.175[29] Bekheet A E. AC conductivity and dielectric properties of Ga2S3-Ga2Se3 films. Physica B, 2008, 403(23/24):4342 http://www.academia.edu/23486129/Progress_on_the_Photoresponse_of_Chalcogenide_Glasses_and_Films_to_Near-Infrared_Femtosecond_Laser_Irradiation_A_Review[30] Amar N M, Saleh A M, Gould R D. Influence of temperature and frequency on the electrical parameters of thermally evaporated metal-free phthalocyanine H2Pc thin films. Appl Phys A, 2003, 76(1):77 doi: 10.1007/s003390201306[31] Nadkarni G S, Simmons J G. Electrical properties of evaporated molybdenum oxide films. J Appl Phys, 1970, 41(2):545 doi: 10.1063/1.1658710[32] Saleh A M, Gould R D, Hassan A K. Dependence of AC electrical parameters on frequency and temperature in zinc phthalocyanine thin films. Phys Status Solidi A, 1993, 139(2):379 doi: 10.1002/(ISSN)1521-396X[33] Riad A S, Korayem M T, Abedl-Malik T G. AC conductivity and dielectric measurements of metal-free phthalocyanine thin films dispersed in polycarbonate. Physica B, 1999, 270(1/2):140 doi: 10.1080/00207219208925582[34] Jarsoz J, Quinn P D, Stephan N, et al. Dielectric properties of zinc phthalocyanine thin films:effects of annealing in air and N2. Thin Solid Films, 2005, 474(1/2):301 https://core.ac.uk/display/30310751[35] Elliott S R. A theory of a.c. conduction in chalcogenide glasses. Philos Magz, 1977, 36(6):1291. Also in, Elliott S R. Temperature dependence of a.c. conductivity of chalcogenide glasses. Philos Magz, 1978, 37(5):553 doi: 10.1080/01418637808226448[36] Jonscher A K. Alternating current diagnostics of poorly conducting thin films. Thin Solid Films, 1976, 369(1):1 doi: 10.1142/7606/suppl_file/7606_chap12.pdf[37] Le Moigne J, Even R. Spectroscopic properties and conductivity of thin films of partially reduced metallo-phthalocyanines. J Chem Phys, 1985, 83(12):6472 doi: 10.1063/1.449547[38] Gould R D, Awan S A. Dielectric properties of AlNx thin films prepared by RF magnetron sputtering of Al using a N2/Ar sputtering gas mixture. Thin Solid Films, 2004, 469/470:184 doi: 10.1016/j.tsf.2004.08.099[39] Mahmood F S, Gould R D. A.C. properties of ZnO thin films prepared by r.f. magnetron sputtering. Thin Solid Films, 1994, 253(1/2):529[40] Vidadi Y, Rozenshtein L D, Chistyokov E A. Hopping and band conductivities in organic semiconductors. Sov Phys:Solid State, 1969, 11(1):173[41] Khan G A, Hogarth C A. Optical absorption spectra of evaporated V2O5 and co-evaporated V2O5/B2O3 thin films. J Mat Sci, 1991, 26(2):412 doi: 10.1007/BF00576535[42] Long A R. Frequency-dependent loss in amorphous semiconductors. Adv Phys, 1982, 31(5):553 doi: 10.1080/00018738200101418 -
Proportional views





 DownLoad:
DownLoad: