| Citation: |
S. P. Yadav, S. S. Shinde, A. A. Kadam, K. Y. Rajpure. Structural, morphological, dielectrical and magnetic properties of Mn substituted cobalt ferrite[J]. Journal of Semiconductors, 2013, 34(9): 093002. doi: 10.1088/1674-4926/34/9/093002
****
S P Yadav, S S Shinde, A A Kadam, K Y Rajpure. Structural, morphological, dielectrical and magnetic properties of Mn substituted cobalt ferrite[J]. J. Semicond., 2013, 34(9): 093002. doi: 10.1088/1674-4926/34/9/093002.
|
Structural, morphological, dielectrical and magnetic properties of Mn substituted cobalt ferrite
DOI: 10.1088/1674-4926/34/9/093002
More Information
-
Abstract
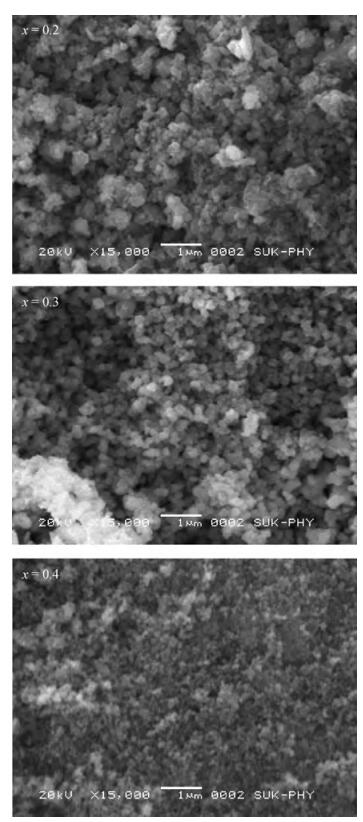
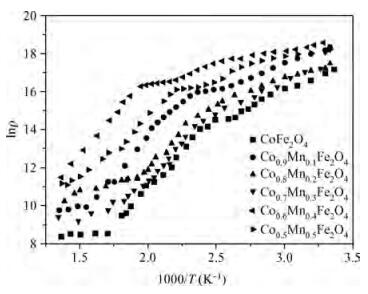
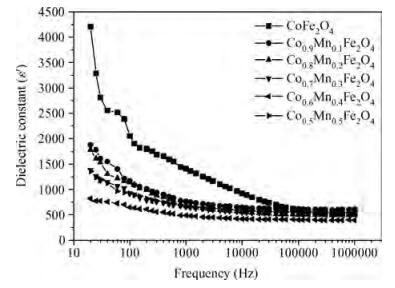
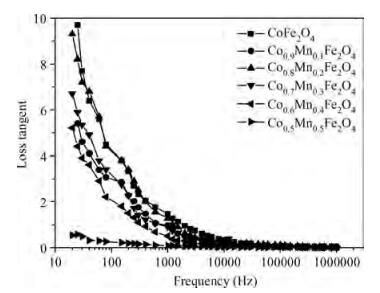
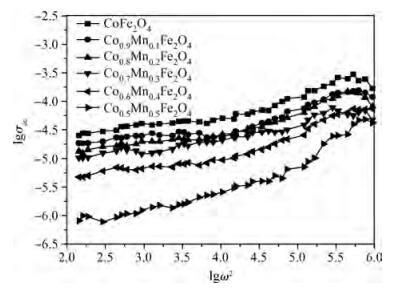
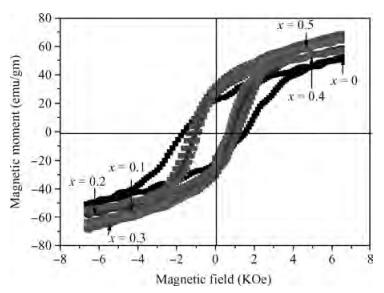
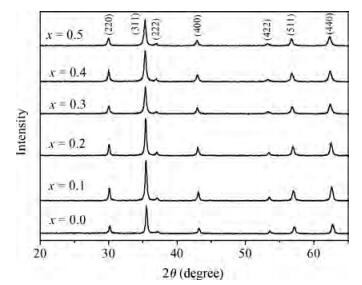
The Co1-xMnxFe2O4 (0 ≤ x ≤ qslant 0.5) ferrite system is synthesized by using an auto combustion technique using metal nitrates. The influence of Mn substitution on the structural, electrical, impedance and magnetic properties of cobalt ferrite is reported. X-ray diffraction patterns of the prepared samples confirm that the Bragg's peak belongs to a spinel cubic crystal structure. The lattice constant of cobalt ferrite increases with the increase in Mn content. The microstructural study is carried out by using the SEM technique and the average grain size continues to increase with increasing manganese content. AC conductivity analysis suggests that the conduction is due to small polaron hopping. DC electrical resistivity decreases with increasing temperature for a Co1-xMnxFe2O4 system showing semiconducting behavior. The activation energy is found to be higher in the paramagnetic region than the ferromagnetic region. Curie temperature decreases with Mn substitution in the host ferrite system. Dielectric dispersion having Maxwell-Wagner-type interfacial polarization has been observed for cobalt ferrite samples. Magnetic properties have been studied by measuring M-H plots. The saturation and remanent magnetization increases with Mn substitution.-
Keywords:
- combustion,
- Mn substitution,
- structural,
- dielectric,
- magnetic
-
References
[1] HaÈfelli U, SchuÈtt W, Teller J, et al. Scientific and clinical applications of magnetic carriers. Plenum, New York, 1997[2] Valenzuela R. Magnetic ceramics. Cambridge:Cambridge University Press, 1984:212[3] Paulsen J A, Lo C C H, Snyder J E, et al. Study of the Curie temperature of cobalt ferrite based composites for stress sensor applications. IEEE Trans Magn, 2003, 39:3316 doi: 10.1109/TMAG.2003.816761[4] Palamaru M N, Iordan A R, Aruxandei C D, et al. The synthesis of doped manganese cobalt ferrites by auto combustion technique. J Optoelec Adv Mater, 2008, 10:1853 doi: 10.1007/s11706-012-0167-3[5] Kim W C, Yi Y S, Kim C S. Structural and magnetic properties of Co-Mn ferrite prepared by a sol-gel method. J Magn, 2000, 5:111[6] Ji J K, Ahn W K, Kum J S, et al. Miniaturized T-DMB antenna with a low-loss Ni-Mn-Co ferrite for mobile handset applications. IEEE Magn Lett, 2010, 1:5000104 doi: 10.1109/LMAG.2009.2038580[7] Moumen N, Veillet P, Pileni M P. Controlled preparation of nanosize cobalt ferrite magnetic particles. J Magn Magn Mater, 1995, 149:67 doi: 10.1016/0304-8853(95)00340-1[8] Micelles R, Seip C T, Carpenter E E, et al. Magnetic properties of a series of ferrite nanoparticles synthesized. IEEE Trans Magn, 1998, 34:1111 doi: 10.1109/20.706388[9] Grigorova M, Blythe H J, Blaskov V, et al. Magnetic properties and Mösbauer spectra of nanosized CoFe2O4 powders. J Magn Magn Mater, 1998, 183:163 doi: 10.1016/S0304-8853(97)01031-7[10] Yan C H, Xu Z G, Cheng F X, et al. Nanophased CoFe2O4 prepared by combustion method. Solid State Commun, 1999, 111:287 doi: 10.1016/S0038-1098(99)00119-2[11] Prakash A S, Khadar A M A, Patil K C, et al. Hexamethylenete-tramine:a new fuel for solution combustion synthesis of complex metal oxides. J Mater Synth Processing, 2002, 10:135 doi: 10.1023/A:1021986613158[12] Bhatu S S, Lakhani V K, Tanna A R, et al. Effect of nickel substitution on structural, infrared and elastic properties of lithium ferrite. Ind J Pure Appl Phys, 2007, 45:596 doi: 10.3103/S1061386214020083[13] McQueeney R J, Bishop A R, Yi Y S, et al. Charge localization and phonon spectra in hole-doped La2NiO4. J Phys Cond Matter, 2000, 12:L317 doi: 10.1088/0953-8984/12/21/102[14] Vasoya N H, Lakhani V K, Sharma P U, et al. Study on the electrical and dielectric behaviour of Zn-substituted cobalt ferrialuminates. J Phys:Condens Matter, 2006, 18:8063 doi: 10.1088/0953-8984/18/34/017[15] Bao J E, Zhou J, Yue Z X, et al. Electrical and magnetic studies of Ba3Co2Fe23-12xMn12xO41 Z-type hexaferrites. Mater Sci Eng B, 2003, 99:98 doi: 10.1016/S0921-5107(02)00428-2[16] Ravinder D, Ravikumar B. A study on elastic behavior of rare earth substituted Mn-Zn ferrites. Mater Lett, 2003, 57:4471 doi: 10.1016/S0167-577X(03)00164-2[17] Pujar R B, Kulakarni S N, Chougule B K. Compositional, temperature and frequency dependence of initial permeability in Zr4+ substituted Mg-Zn ferrites. Mater Sci Lett, 1996, 15:1605 http://www.ingentaconnect.com/content/klu/jmsl/1997/00000016/00000020/00174652[18] Zemansky M W. Heat and thermodynamics. 6th ed. New York:Mc Graw-Hill Book Company, 1981[19] Caltun O, Rao G S N, Rao K H, et al. The influence of Mn doping level on magnetostriction coefficient of cobalt ferrite. J Magn Magn Mater, 2006, 316:e618[20] Maxwell J C. Electricity and magnetism. London:Oxford University Press, 1993:828[21] Wagner K W. Ann Phys, 1913, 40: 817[22] Koop C G. On the dispersion of resistivity and dielectric constant of some semiconductors at audio frequencies. Phys Rev B, 1951, 83:121 doi: 10.1103/PhysRev.83.121[23] Devan R S, Kolekar Y D, Chougule B K. Effect of cobalt substitution on the properties of nickel-copper ferrite. J Phys:Conden Matter, 2006, 18:9809 doi: 10.1088/0953-8984/18/43/004[24] Agrawal D C. Asian J Phys, 1997, 6: 108[25] Mahajan R P, Patankar K K, Kothale M B, et al. Conductivity, dielectric behaviour and magnetoelectric effect in copper ferrite-barium titanate composites. Bull Mater Sci, 2000, 23:273 doi: 10.1007/BF02720082 -
Proportional views





 DownLoad:
DownLoad: