| Citation: |
J. V. Thombare, M. C. Rath, S. H. Han, V. J. Fulari. The effects of electron irradiation on the optical properties of the organic semiconductor polypyrrole[J]. Journal of Semiconductors, 2013, 34(9): 093001. doi: 10.1088/1674-4926/34/9/093001
****
J V Thombare, M C Rath, S H Han, V J Fulari. The effects of electron irradiation on the optical properties of the organic semiconductor polypyrrole[J]. J. Semicond., 2013, 34(9): 093001. doi: 10.1088/1674-4926/34/9/093001.
|
The effects of electron irradiation on the optical properties of the organic semiconductor polypyrrole
DOI: 10.1088/1674-4926/34/9/093001
More Information
-
Abstract
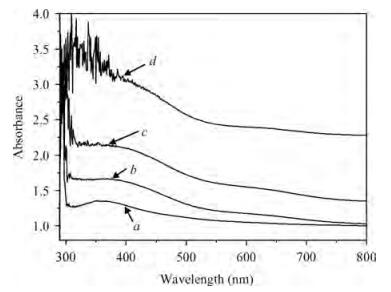
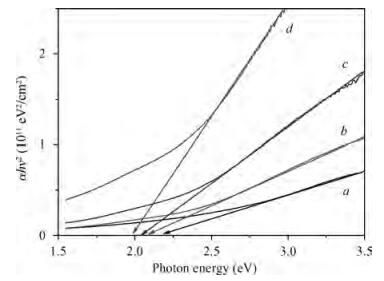
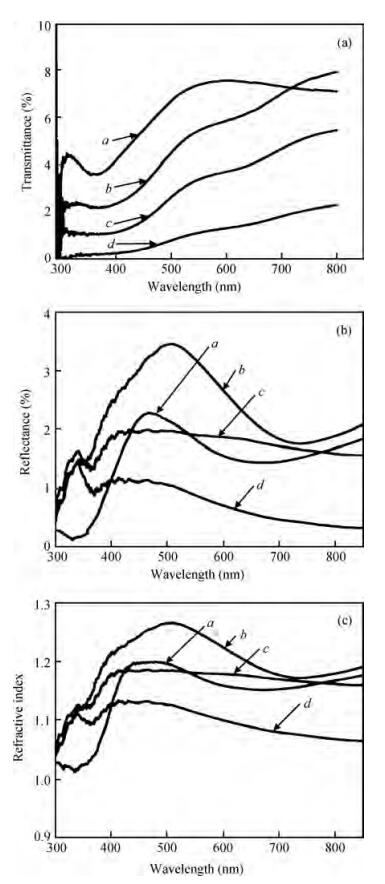
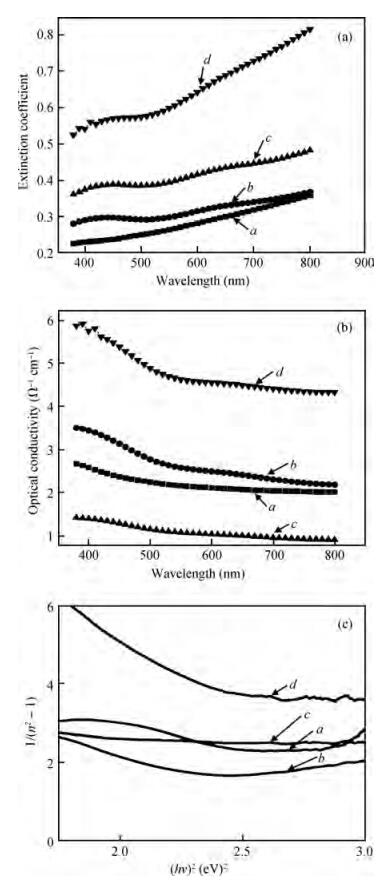
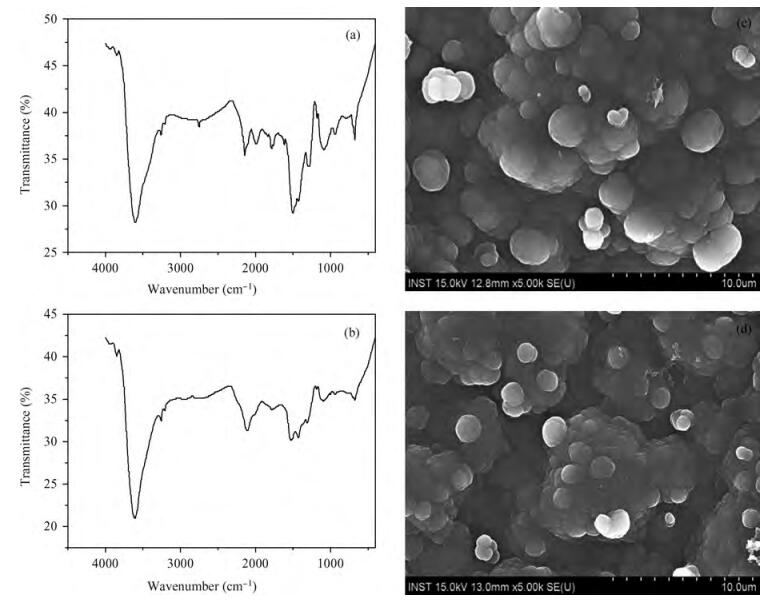
The optical properties of polypyrrole (Ppy) thin films upon 2 MeV electron beam irradiation changes with different doses. The induced changes in the optical properties for Ppy thin films were studied in the visible range 300 to 800 nm at room temperature. The optical band gap of the pristine Ppy was found to be 2.19 eV and it decreases up to 1.97 eV for a 50 kGy dose of 2 MeV electron beam. The refractive index dispersion of the samples obeys the single oscillator model. The obtained results suggest that electron beam irradiation changes the optical parameters of Ppy thin films.-
Keywords:
- polypyrrole,
- electron beam irradiation,
- optical band gap
-
References
[1] Friend R H, Gymer R W, Holmes A B, et al. Electroluminescence in conjugated polymers. Nature, 1999, 397:121 doi: 10.1038/16393[2] White H S, Kittlesen G P, Wrighton M S. Chemical derivatization of an array of three gold microelectrodes with polypyrrole:fabrication of molecular based transistor. J Am Chem Soc, 1984, 106:5375 doi: 10.1021/ja00330a070[3] Kittlesen G P, White H S, Wrighton M S. Chemical derivativization of microelectrode arrays by oxidation of pyrrole and n-methylpyrrole:fabrication of molecule-based electronic devices. J Am Chem Soc, 1984, 106:7389 doi: 10.1021/ja00336a016[4] Ansari R. Polypyrrole conducting electroactive polymers:synthesis and stability studies. J Chem, 2006, 3:186 http://www.oalib.com/paper/2778144[5] Dong H, Cao X, Li C M. Functionalized polypyrrole film:synthesis, characterization, and potential applications in chemical and biological sensors. Appl Mater Inter, 2009, 1:1599 doi: 10.1021/am900267e[6] Lee A S, Peteu S F, Ly J V, et al. Actuation of polypyrrole nanowires. Nanotechnology, 2008, 19:165501 doi: 10.1088/0957-4484/19/16/165501[7] Geetha S, Rao C R K, Vijayan M, et al. Biosensing and drug delivery by polypyrrole. Anal Chim Acta, 2006, 568:119 doi: 10.1016/j.aca.2005.10.011[8] Janata J, Josowicz M. Conducting polymers in electronic chemical sensors. Nat Mater, 2003, 2:19 doi: 10.1038/nmat768[9] Ramanavicius A, Ramanaviciene A, Malinauskas A. Electrochemical sensors based on conducting polymer——polypyrrole. Electrochim Acta, 2006, 51:6025 doi: 10.1016/j.electacta.2005.11.052[10] Zang J, Li X. In situ synthesis of ultrafine β -MnO2/polypyrrole nanorod composites for high-performance supercapacitors. J Mater Chem, 2011, 21:10965 doi: 10.1039/c1jm11491c[11] Li F, Winnik M A, Matvienkob A, et al. Polypyrrole nanoparticles as a thermal transducer of NIR radiation in hot-melt adhesives. J Mater Chem, 2007, 17:4309 doi: 10.1039/b708707a[12] Hong Y, Park D, Jo S, et al. Fine characteristics tailoring of organic and inorganic nanowires using focused electron-beam irradiation. Angew Chem Int Ed, 2011, 50:3734 doi: 10.1002/anie.v50.16[13] Sabharwal S, Mohan H, Bhardwaj Y K, et al. Structure-reactivity studies on the crosslinking of poly (vinyl methyl ether) in aqueous solutions:a pulse radiolysis study. J Chem Soc Faraday Trans, 1996, 92:4401 doi: 10.1039/FT9969204401[14] Dias H V R, Fianchini M, Rajapakse R M G. Greener method for high-quality polypyrrole. Polymer, 2006, 47:7349 doi: 10.1016/j.polymer.2006.08.033[15] Kang H C, Geckeler K E. Enhanced electrical conductivity of polypyrrole prepared by chemical oxidative polymerization:effect of the preparation technique and polymer additive. Polymer, 2000, 41:6931 doi: 10.1016/S0032-3861(00)00116-6[16] Cruz G J, Olayo M G, López O G, et al. Nanospherical particles of polypyrrole synthesized and doped by plasma. Polymer, 2010, 51:4314 doi: 10.1016/j.polymer.2010.07.024[17] Zhou X J, Leung K T. Modification of electronic structure of mesoscopic perchlorate-doped polypyrrole films by ion irradiation. Macromolecules, 2003, 36:2882 doi: 10.1021/ma026023a[18] Foroughi J, Spinks G M, Wallace G G. A reactive wet spinning approach to polypyrrole fibers. J Mater Chem, 2011, 21:6421 doi: 10.1039/c0jm04406g[19] Fu Y, Manthiram A. Enhanced cyclability of lithium-sulfur batteries by a polymer acid-doped polypyrrole mixed ionic-electronic conductor. Chem Mater, 2012, 24:3081 doi: 10.1021/cm301661y[20] Chandra S, Annapoorni S, Singh F, et al. Low temperature resistivity study of nanostructured polypyrrole films under electronic excitations. Nucl Instr & Methods B, 2010, 62:268[21] Nadeem M Y, Ahmed W. Optical properties of ZnS thin films. Turk J Phys, 2000, 24:651 http://www.jnus.org/pdf/2011/6/88.pdf[22] Wemple S H, Domenico M D Jr. Behavior of the electronic dielectric constant in covalent and ionic materials. Phys Rev B, 1971, 3:1338 doi: 10.1103/PhysRevB.3.1338[23] Bhattacharyya S R, Gayen R N, Paul R, et al. Determination of optical constants of thin films from transmittance trace. Thin Solid Films, 2009, 517:5530 doi: 10.1016/j.tsf.2009.03.168 -
Proportional views





 DownLoad:
DownLoad: