| Citation: |
Rui Chen, Jin Kang, Yuling Liu, Chenwei Wang, Ting Cai, Xin Li. A new weakly alkaline slurry for copper planarization at a reduced down pressure[J]. Journal of Semiconductors, 2014, 35(2): 026005. doi: 10.1088/1674-4926/35/2/026005
****
R Chen, J Kang, Y L Liu, C W Wang, T Cai, X Li. A new weakly alkaline slurry for copper planarization at a reduced down pressure[J]. J. Semicond., 2014, 35(2): 026005. doi: 10.1088/1674-4926/35/2/026005.
|
A new weakly alkaline slurry for copper planarization at a reduced down pressure
DOI: 10.1088/1674-4926/35/2/026005
More Information
-
Abstract
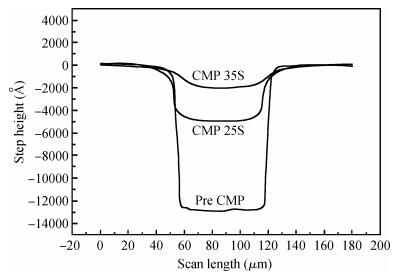
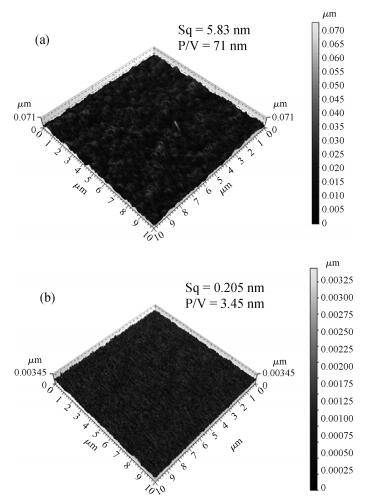
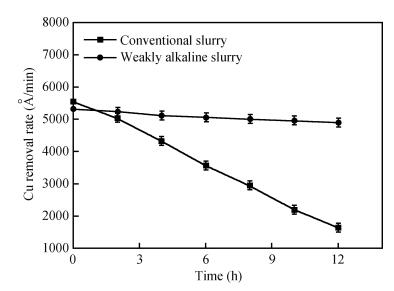
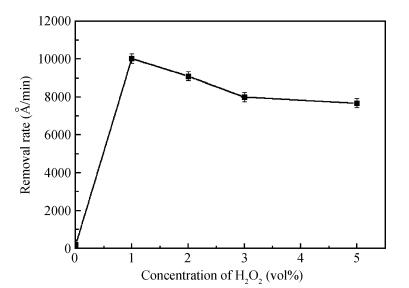
This study reports a new weakly alkaline slurry for copper chemical mechanical planarization (CMP), it can achieve a high planarization efficiency at a reduced down pressure of 1.0 psi. The slurry is studied through the polish rate, planarization, copper surface roughness and stability. The copper polishing experiment result shows that the polish rate can reach 10032 Å/min. From the multi-layers copper CMP test, a good result is obtained, that is a big step height (10870 Å) that can be eliminated in just 35 s, and the copper root mean square surface roughness (sq) is very low ( < 1 nm). Apart from this, compared with the alkaline slurry researched before, it has a good progress on stability of copper polishing rate, stable for 12 h at least. All the results presented here are relevant for further developments in the area of copper CMP. -
References
[1] Pandija S, Roy D, Babu V S. Achievement of high planarization efficiency in CMP of copper at a reduced down pressure. Microelectron Eng, 2009, 86(3):367 doi: 10.1016/j.mee.2008.11.047[2] Pandija S, Roy D, Babu S V. Chemical mechanical planarization of copper using abrasive-free solutions of oxalic acid and hydrogen peroxide. Mater Chem Phys, 2007, 102(2/3):144 http://cat.inist.fr/?aModele=afficheN&cpsidt=18617785[3] Liu X Y, Liu Y L, Liang Y, et al. Effect of slurry components on chemical mechanical polishing of copper at low down pressure and a chemical kinetics model. Thin Solid Films, 2011, 520(1):400 doi: 10.1016/j.tsf.2011.06.050[4] Prasad Y N, Ramanathan S. Chemical mechanical planarization of copper in alkaline slurry with uric acid as inhibitor. Electrochimica Acta, 2007, 52(22):6353 doi: 10.1016/j.electacta.2007.04.044[5] Mahadevaiyer K, Jakub W, Lee M. Chemical mechanical planarization:slurry chemistry, materials and mechanisms. Chem Rev, 2010, 110(1):179 doi: 10.1021/cr900170z?src=recsys&[6] Hegde S, Partri U B, Babu S V. Chemical-mechanical polishing of copper using molybdenum dioxide slurry. J Mater Research, 2005, 20(9):2553 doi: 10.1557/jmr.2005.0305[7] Wang C W, Liu Y L, Tian J Y. Planarization properties of an alkaline slurry without an inhibitor on copper patterned wafer CMP. Journal of Semiconductors, 2012, 33(11):116001 doi: 10.1088/1674-4926/33/11/116001[8] Oh Y J, Park G S, Chung C H. Planarization of copper layer for damascene interconnection by electrochemical polishing in alkali-based solution. J Electrochem Soc, 2006, 153(7):G617 doi: 10.1149/1.2200288[9] Yin K D, Wang S L, Liu Y L. Evaluation of planarization capability of copper slurry in the CMP process. Journal of Semiconductors, 2013, 34(3):036002 doi: 10.1088/1674-4926/34/3/036002[10] Janjam S V S B, Peethala B C, Zheng J P. Electrochemical investigation of surface reaction for chemically promoted chemical mechanical polishing of TaN in tartaric acid solutions. Mater Chem Phys, 2010, 123(2/3):521 http://www.sciencedirect.com/science/article/pii/S0254058410003755[11] Qin K, Moudgil B, Park C W. A chemical mechanical polishing model incorporating both the chemical and mechanical effects. Thin Solid Films, 2004, 446(2):277 doi: 10.1016/j.tsf.2003.09.060 -
Proportional views





 DownLoad:
DownLoad: