| Citation: |
Yan Li, Yuling Liu, Aochen Wang, Zhixin Yang, Mingbin Sun, Chuan Cheng, Yufeng Zhang, Nannan Zhang. The application of Cu/SiO2 catalytic system in chemical mechanical planarization based on the stability of SiO2 sol[J]. Journal of Semiconductors, 2014, 35(6): 066002. doi: 10.1088/1674-4926/35/6/066002
****
Y Li, Y L Liu, A C Wang, Z X Yang, M B Sun, C Cheng, Y F Zhang, N N Zhang. The application of Cu/SiO2 catalytic system in chemical mechanical planarization based on the stability of SiO2 sol[J]. J. Semicond., 2014, 35(6): 066002. doi: 10.1088/1674-4926/35/6/066002.
|
The application of Cu/SiO2 catalytic system in chemical mechanical planarization based on the stability of SiO2 sol
DOI: 10.1088/1674-4926/35/6/066002
More Information
-
Abstract
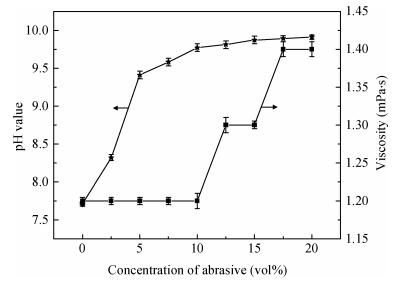
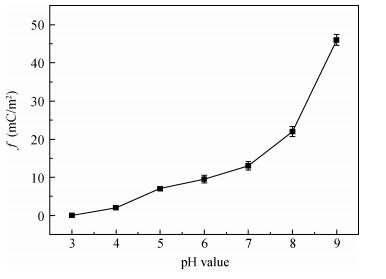
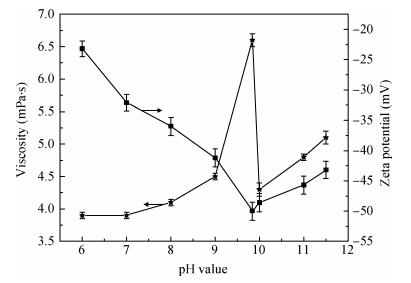
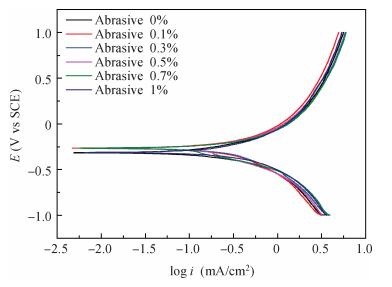
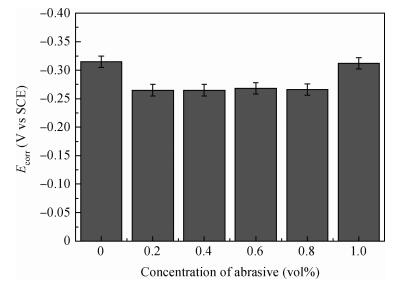
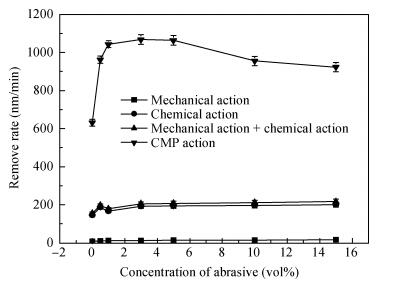
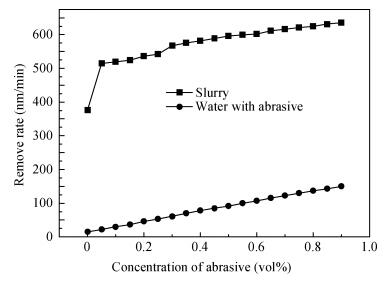
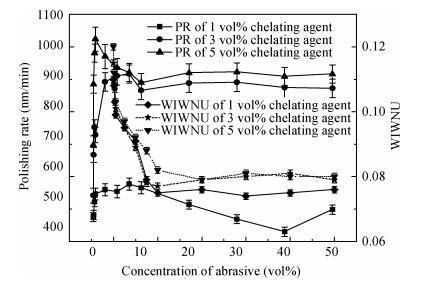
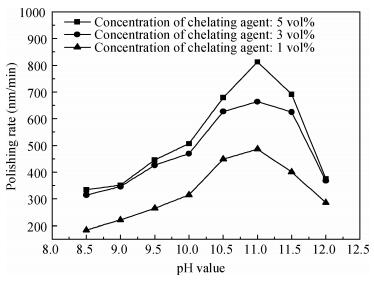
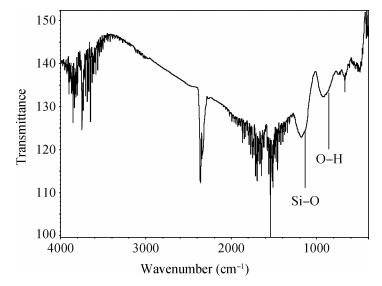
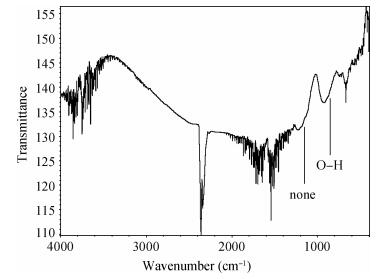
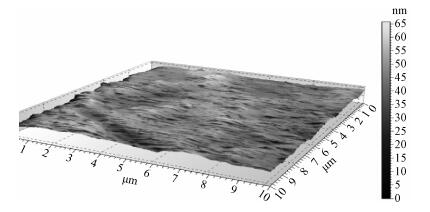
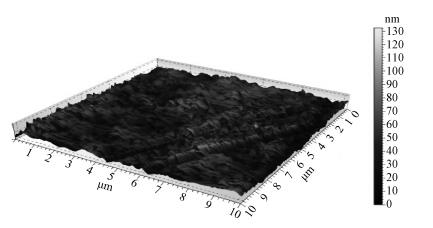
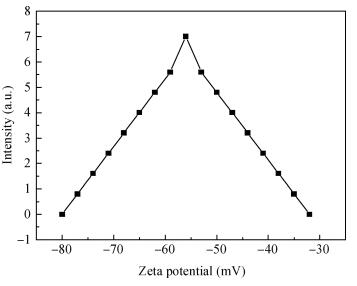
There is a lot of hydroxyl on the surface of nano SiO2 sol used as an abrasive in the chemical mechanical planarization (CMP) process, and the chemical reaction activity of the hydroxyl is very strong due to the nano effect. In addition to providing a mechanical polishing effect, SiO2 sol is also directly involved in the chemical reaction. The stability of SiO2 sol was characterized through particle size distribution, zeta potential, viscosity, surface charge and other parameters in order to ensure that the chemical reaction rate in the CMP process, and the surface state of the copper film after CMP was not affected by the SiO2 sol. Polarization curves and corrosion potential of different concentrations of SiO2 sol showed that trace SiO2 sol can effectively weaken the passivation film thickness. In other words, SiO2 sol accelerated the decomposition rate of passive film. It was confirmed that the SiO2 sol as reactant had been involved in the CMP process of copper film as reactant by the effect of trace SiO2 sol on the removal rate of copper film in the CMP process under different conditions. In the CMP process, a small amount of SiO2 sol can drastically alter the chemical reaction rate of the copper film, therefore, the possibility that Cu/SiO2 as a catalytic system catalytically accelerated the chemical reaction in the CMP process was proposed. According to the van't Hoff isotherm formula and the characteristics of a catalyst which only changes the chemical reaction rate without changing the total reaction standard Gibbs free energy, factors affecting the Cu/SiO2 catalytic reaction were derived from the decomposition rate of Cu (OH)2 and the pH value of the system, and then it was concluded that the CuSiO3 as intermediates of Cu/SiO2 catalytic reaction accelerated the chemical reaction rate in the CMP process. It was confirmed that the Cu/SiO2 catalytic system generated the intermediate of the catalytic reaction (CuSiO3) in the CMP process through the removal rate of copper film, infrared spectrum and AFM diagrams in different pH conditions. Finally it is concluded that the SiO2 sol used in the experiment possesses stable performance; in the CMP process it is directly involved in the chemical reaction by creating the intermediate of the catalytic reaction (CuSiO3) whose yield is proportional to the pH value, which accelerates the removal of copper film.-
Keywords:
- CMP,
- SiO2 sol,
- zeta potential,
- CuSiO3,
- polarization curves
-
References
[1] Sulyma C M, Roy D. Electrochemical characterization of surface complexes formed on Cu and Ta in succinic acid based solutions used for chemical mechanical planarization. Appl Surf Sci, 2010, 256:2583 doi: 10.1016/j.apsusc.2009.10.108[2] Oh S, Seok J. An integrated material removal model for silicon dioxide layers in chemical mechanical polishing processes. Wear, 2009, 266(7/8):839 http://www.sciencedirect.com/science/article/pii/S0043164808004328[3] Tsai T C, Tsao W C, Lin W, et al. CMP process development for the via-middle 3D TSV applications at 28 nm technology node. Microeletron Eng, 2012, 92(3):29 http://dl.acm.org/citation.cfm?id=2169899[4] Liu Y L, Tan B M, Zhang K L. Microelectronic technology engineering. Beijing:Electronics Industry Press, 2004[5] Zheng J P, Roy D. Electrochemical examination of surface films formed during chemical mechanical planarization of copper in acetic acid and dodecyl sulfate solutions. Thin Solid Films, 2009, 517(16):4587 doi: 10.1016/j.tsf.2009.03.063[6] Yang J C, Oh D W, Lee G W, et al. Step height removal mechanism of chemical mechanical planarization (CMP) for sub-nano-surface finish. Wear, 2010, 268(3/4):505 http://www.academia.edu/12683789/Step_height_removal_mechanism_of_chemical_mechanical_planarization_CMP_for_sub-nano-surface_finish[7] Wang X, Liu Y L, Wang H Y. The analysis on mechanical action of abrasive particles in Cu-CMP. Electron Device, 2002, (09):87 http://en.cnki.com.cn/Article_en/CJFDTOTAL-DZQJ200203002.htm[8] Zhang K L, Song Z T, Zhang J X. Study on the synthesis and application of large particle size silica sol used as nano abrasive in the CMP process of medium. Electron Device, 2004, 27(04):556[9] Zhang L, Wang H B, Zhang Z F, et al. Research of chemical mechanical polishing on copper by a novel composite abrasive. Journal of Functional Materials and Devices, 2011, 05:520[10] Lei H, Luo J J. CMP of hard disk substrate using a colloidal SiO2 slurry:preliminary experimental investigation. Wear, 2004, 257(5/6):461 http://www.sciencedirect.com/science/article/pii/S004316480400016X[11] Shattuck K G, Lin J Y, Cojocaru P, et al. Characterization of phosphate electrolytes for use in Cu electrochemical mechanical planarization. Electrochemical Acta, 2008, 53:8211 doi: 10.1016/j.electacta.2008.05.077[12] Noh K, Saka N, Chun J H. Effect of slurry selectivity on dielectric erosion and copper dishing in copper chemical-mechanical polishing. CIRP Annals-Manufacturing Technology, 2004, 53(1):463 doi: 10.1016/S0007-8506(07)60740-9[13] Hu Yi, Liu Yuling, Liu Xiaoyan, et al. Effect of copper slurry on polishing characteristics. Journal of Semiconductors, 2011, 32(11):116001 doi: 10.1088/1674-4926/32/11/116001[14] Hu Yi, Liu Yuling, Liu Xiaoyan, et al. Effect of alkaline slurry on the electric character of the pattern Cu wafer. Journal of Semiconductors, 2011, 32(7):076002 doi: 10.1088/1674-4926/32/7/076002[15] Pandija S, Roy D, Babu S V. Achievement of high planarization efficiency in CMP of copper at a reduced down pressure. Microelectron Eng, 2009, 86:367 doi: 10.1016/j.mee.2008.11.047[16] Zhang W, Lu X C, Liu Y H, et al. Inhibitors for organic phosphonic acid system abrasive free polishing of Cu. Appl Surf Sci, 2009, 255:4114 doi: 10.1016/j.apsusc.2008.10.096[17] Weng T T, Mishra A, Guo Y J. Regulation of lung surfactant secretion by microRNA-150. Biochemical and Biophysical Research Communications, 2012, 422:586 doi: 10.1016/j.bbrc.2012.05.030[18] Ellen R P, Michael S. Simulation of spreading surfactant on a thin liquid film. Applied Mathematics and Computation, 2012, 218:5157 doi: 10.1016/j.amc.2011.11.002[19] Wioletta O S, Katarzyna D, Dawid S. Densities and viscosities of imidazolium and pyridinium chloroaluminate ionic liquids. Journal of Molecular Liquids, 2013, 177:85 doi: 10.1016/j.molliq.2012.10.001[20] Farhad G, Poorandokht I K, Amir H M, et al. Development of a group contribution method for determination of viscosity of ionic liquids at atmospheric pressure. Chem Eng Sci, 2012, 80:326 doi: 10.1016/j.ces.2012.06.045[21] Junji M, Shun S, Takeshi O. Structural and chemical characteristics of atomically smooth GaN surfaces prepared by abrasive-free polishing with Pt catalyst. J Cryst Growth, 2012, 349:83 doi: 10.1016/j.jcrysgro.2012.04.007[22] Ng D, Kulkarni M, Johnson J. Oxidation and removal mechanisms during chemical-mechanical planarization. Wear, 2007, 263:1477 doi: 10.1016/j.wear.2006.11.023 -
Proportional views





 DownLoad:
DownLoad: