| Citation: |
Jing Xu, Yuelin Wei, Yunfang Huang, Jing Wang, A'xiang Chen, Leqing Fan, Jihuai Wu. Microwave-assisted synthesis of Mo-doped H1-xSr2Nb3-xMoxO10 (x=0, 0.05, 0.1, 0.15 and 0.2) with high photocatalytic activity[J]. Journal of Semiconductors, 2014, 35(8): 083001. doi: 10.1088/1674-4926/35/8/083001
****
J Xu, Y L Wei, Y F Huang, J Wang, A Chen, L Q Fan, J H Wu. Microwave-assisted synthesis of Mo-doped H1-xSr2Nb3-xMoxO10 (x=0, 0.05, 0.1, 0.15 and 0.2) with high photocatalytic activity[J]. J. Semicond., 2014, 35(8): 083001. doi: 10.1088/1674-4926/35/8/083001.
|
Microwave-assisted synthesis of Mo-doped H1-xSr2Nb3-xMoxO10 (x=0, 0.05, 0.1, 0.15 and 0.2) with high photocatalytic activity
DOI: 10.1088/1674-4926/35/8/083001
More Information
-
Abstract
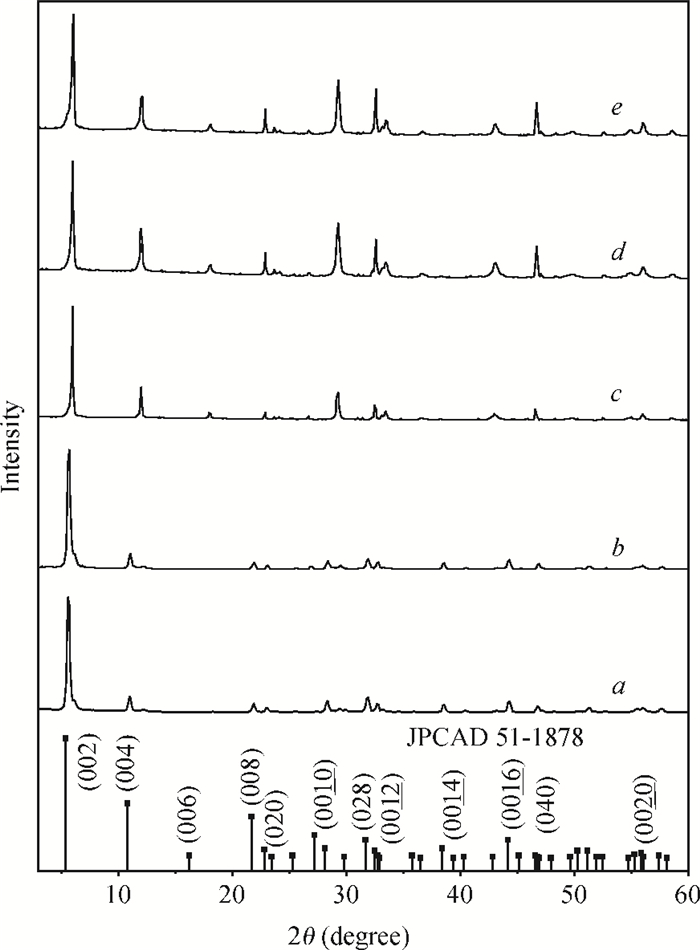
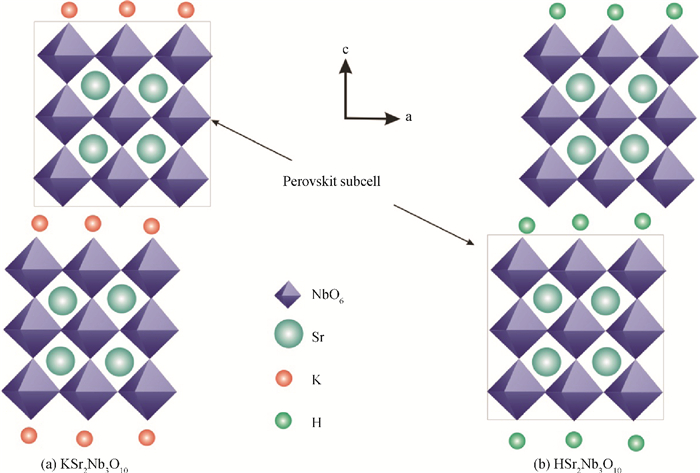
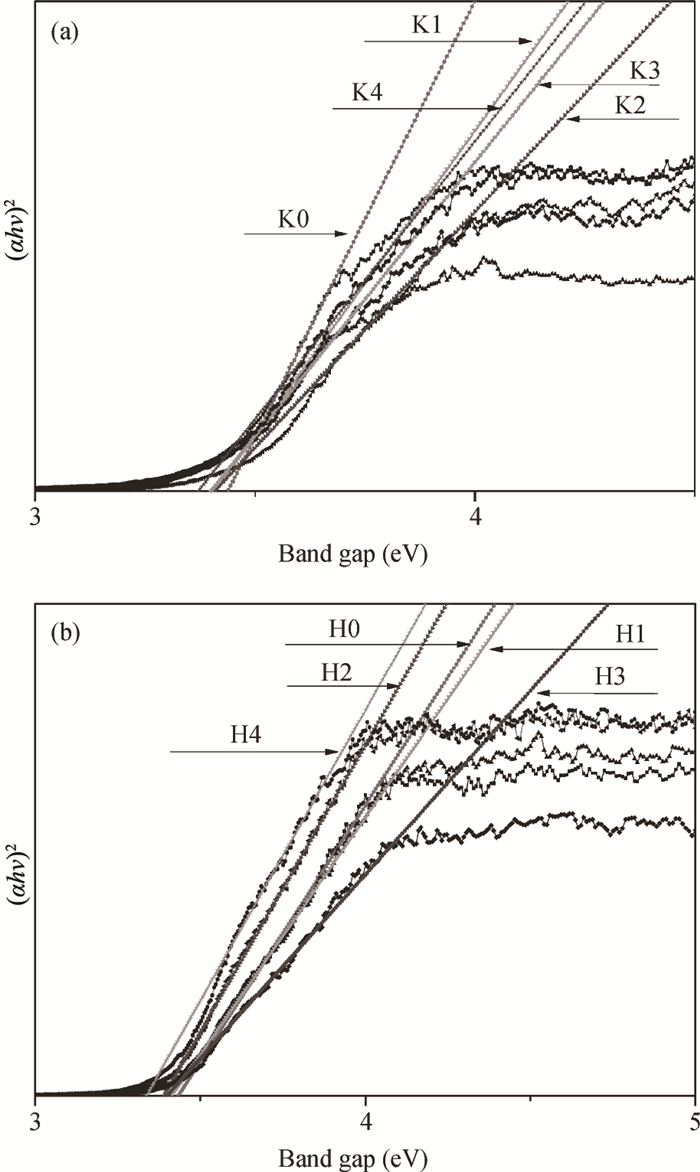
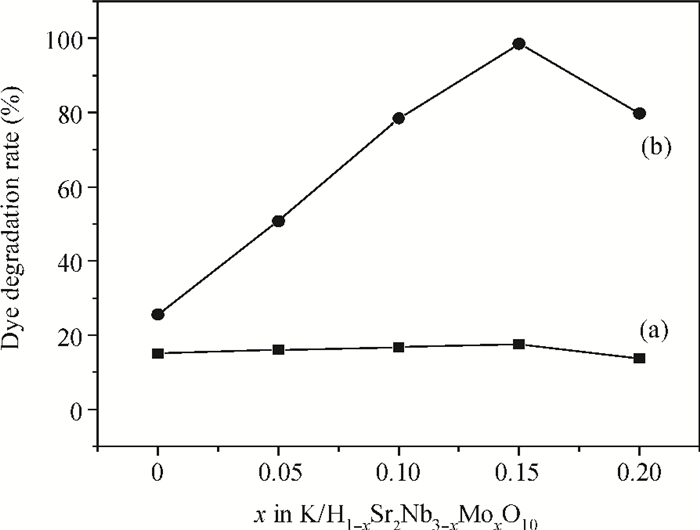
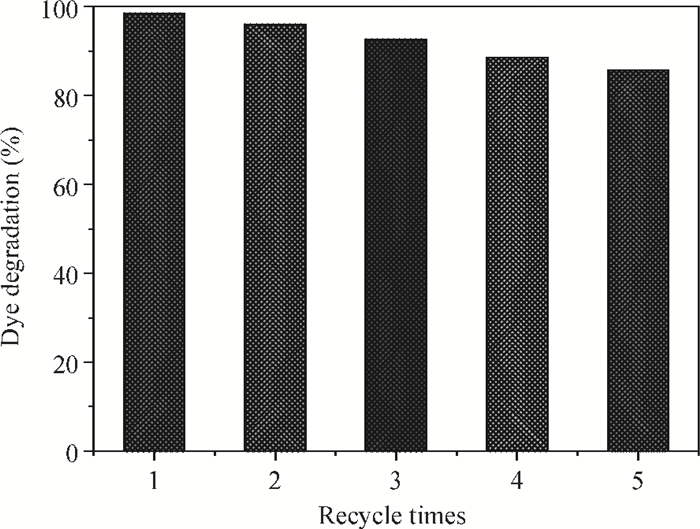
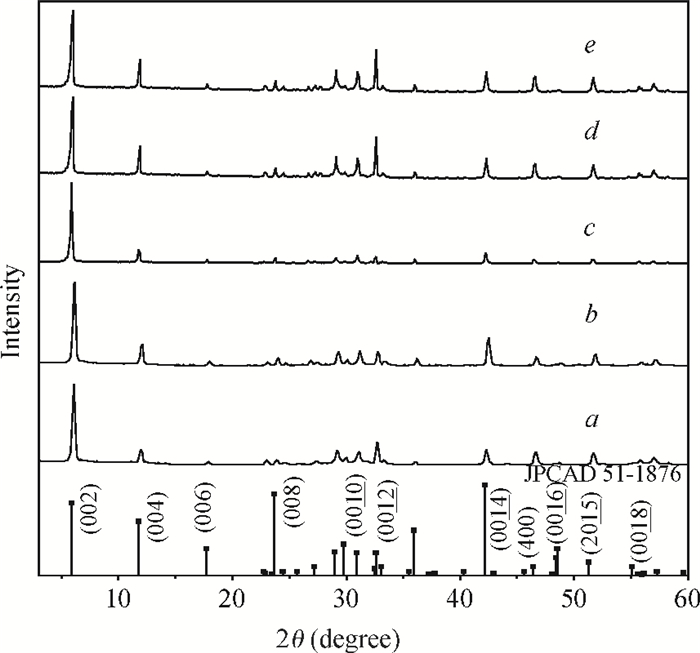
H1-xSr2Nb3-xMoxO10 photocatalysts were synthesized using a microwave-irradiation-assisted ion-exchange reaction. The physicochemical properties of the photocatalysts were analyzed by field-emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD) and ultraviolet-visible spectroscopy (UV-Vis). The photocatalytic activity of the H1-xSr2Nb3-xMoxO10 was evaluated by degrading methyl orange (MO) dye under light irradiation from a 40-W mercury lamp. The results proved that the Mo doping amount had an important effect on the photocatalytic activity of the catalysts. The highest photocatalytic activity was obtained when the doping amount was 15 mol%. Furthermore, the complex preparation process and lengthy time was simplified and shortened greatly.-
Keywords:
- photocatalysis,
- H1-xSr2Nb3-xMoxO10,
- microwave-assisted
-
References
[1] Chen X B, Shen S H, Guo L J, et al. Semiconductor-based photocatalytic hydrogen generation. Chem Rev, 2010, 110:6503 doi: 10.1021/cr1001645[2] Kudo A, Miseki Y. Heterogeneous photocatalyst materials for water splitting. Chem Soc Rev, 2009, 38:253 doi: 10.1039/B800489G[3] Shinde S S, Bhosale C H, Rajpure K Y. Solar light assisted photocatalysis of water using a zinc oxide semiconductor. J Semicomd, 2013, 34:043002 http://kns.cnki.net/KCMS/detail/detail.aspx?filename=bdtx201304005&dbname=CJFD&dbcode=CJFQ[4] Sapkal R T, Shinde S S, Rajpure K Y, Photoelectrocatrocatalytic hydrolysis of starch by using sprayed ZnO thin films. J Semicomd, 2013, 34:053001 http://kns.cnki.net/KCMS/detail/detail.aspx?filename=bdtx201305005&dbname=CJFD&dbcode=CJFQ[5] Gopalakrishnan J, Bhat V. A2Ln2Ti3O10 (A = K or Rb; Ln = La or rare earth):a new series of layered perovskites exhibiting ion exchange. Inorg Chem, 1987, 26:4299 doi: 10.1021/ic00273a001[6] Uma S, Raju AR, Gopalakrishnan J. Bridging the Ruddlesden-Popper and the Dion-Jacobson series of layered perovskites:synthesis of layered oxides, A2-xLa2Ti3-xNbxO10 (A = K, Rb), exhibiting ion exchange. J Mater Chem, 1993, 3:709 doi: 10.1039/JM9930300709[7] Machida M, Mitsuyama T, Ikeue K, et al. Photocatalytic property and electronic structure of triple-layered perovskite tantalates, MCa2Ta3O10 (M = Cs, Na, H, and C6H13NH3). J Phys Chem B, 2005, 109:7801 doi: 10.1021/jp044833d[8] Wang J S, Yin S, Sato T. Characterization of H2Ti4O9 with high specific surface area prepared by a delamination/reassembling process. Mater Sci Eng B, 2006, 126:53 doi: 10.1016/j.mseb.2005.08.083[9] Sarahan M C, Carroll E C, Allen M, et al. K4Nb6O17-derived photocatalysts for hydrogen evolution from water:nanoscrolls versus nanosheets. J Solid State Chem, 2008, 181:1678 doi: 10.1016/j.jssc.2008.06.021[10] Tai Y W, Chen J S, Yang C C, et al. Preparation of nano-gold on K2La2Ti3O10 for producing hydrogen from photo-catalytic water splitting. Cat Today, 2004, 97:95 doi: 10.1016/j.cattod.2004.04.054[11] Wu J H, Cheng Y H, Lin J M, et al. Fabrication and photocatalytic properties of HLaNb2O7/(Pt, Fe2O3) pillared nanomaterial. J Phys Chem C, 2007, 111:3624 doi: 10.1021/jp0674930[12] Kleiman-Shwarsctein A, Hu Y S, Forman A J, et al. Electrodeposition of α-Fe2O3 doped with Mo or Cr as photoanodes for photocatalytic water splitting. J Phys Chem C, 2008, 112:15900 doi: 10.1021/jp803775j[13] Zong X, Yan H J, Wu G P, et al. Enhancement of photocatalytic H2 evolution on CdS by loading MoS2 as cocatalyst under visible light irradiation. J Am Chem Soc, 2008, 130:7176 doi: 10.1021/ja8007825[14] Joshi U A, Jang J S, Borse P H, et al. Microwave synthesis of single-crystalline perovskite BiFeO3 nanocubes for photoelectrode and photocatalytic applications. Appl Phys Lett, 2008, 92:242106 doi: 10.1063/1.2946486[15] Wu H Q, Wang Q Y, Yao Y Z, et al. Microwave-assisted synthesis and photocatalytic properties of carbon nanotube/zinc sulfide heterostructures. J Phys Chem C, 2008, 112:16779 doi: 10.1021/jp8069018[16] Zhang P L, Yin S, Sato T. Synthesis of high-activity TiO2 photocatalyst via environmentally friendly and novel microwave assisted hydrothermal process. Appl Catal B:Environ, 2009, 89:118 doi: 10.1016/j.apcatb.2008.12.002[17] Liu X G, Geng D Y, Wang X L, et al. Enhanced photocatalytic activity of Mo-{001}TiO2 core-shell nanoparticles under visible light. Chem Commun, 2010, 46:6956 doi: 10.1039/c0cc02034f[18] Xu Z C, Li Q, Gao S A, et al. Synthesis and characterization of niobium-doped TiO2 nanotube arrays by anodization of Ti20Nb Alloys. J Mater Sci Technol, 2012, 28:865 doi: 10.1016/S1005-0302(12)60144-3[19] Feng L L, Zou X X, Zhao J, et al. Nanoporous Sr-rich strontium titanate:a stable andsuperior photocatalyst for H2 evolution. Chem Commun, 2013, 49:9788 doi: 10.1039/c3cc45795h[20] Hu Y C, Guo P H, Guo L J. Synthesis and photocatalytic properties of Cr-doped KSr2Nb3O10 for hydrogen production. Int J Hydrogen Energy, 2012, 37:1007 doi: 10.1016/j.ijhydene.2011.03.050[21] Maeda K, Mallouk T E. Comparison of two-and three-layer restacked Dion-Jacobson phase niobate nanosheets as catalysts for photochemical hydrogen evolution. J Mater Chem, 2009, 19:4813 doi: 10.1039/b903692j[22] Gao G, Rabenberg L K, Nunn C M, et al. Formation of quantum-size semiconductor particles in a layered metal phosphonate host lattice. Chem Mater, 1991, 3:149 doi: 10.1021/cm00013a032[23] Linsebigler A L, Lu G Q, Yates J T. Photocatalysis on TiO2 surfaces:principles, mechanisms, and selected results. Chem Rev, 1995, 95:735 doi: 10.1021/cr00035a013[24] Gao P, Zhang X J, Zhou W F, et al. First-principle study on anatase TiO2 codoped with nitrogen and ytterbium. J Semicomd, 2010, 3:032001 http://kns.cnki.net/KCMS/detail/detail.aspx?filename=bdtx201003003&dbname=CJFD&dbcode=CJFQ[25] Pleskov Y V. Conversion of luminous energy into electrical and chemical energy in photoelectrochemical cells with semiconductor electrodes. Sov Electrochem, 1981, 17:1 https://www.researchgate.net/publication/279611302_CONVERSION_OF_LUMINOUS_ENERGY_INTO_ELECTRICAL_AND_CHEMICAL_ENERGY_IN_PHOTOELECTROCHEMICAL_CELLS_WITH_SEMICONDUCTOR_ELECTRODES_REVIEW[26] Takata T, Furumi Y, Shinohara K, et al. Photocatalytic decomposition of water on spontaneously hydrated layered perovskites. Chem Mater, 1997, 9:1063 doi: 10.1021/cm960612b[27] Zhou J K, Zhang Y X, Zhao X S. Photodegradation of benzoic acid over metal-doped TiO2. Ind Eng Chem Res, 2006, 45:3503 doi: 10.1021/ie051098z -
Proportional views





 DownLoad:
DownLoad: