| Citation: |
Xiaoyan Shen, Zhigong Wang. Fully integrated circuit chip of microelectronic neural bridge[J]. Journal of Semiconductors, 2014, 35(9): 095011. doi: 10.1088/1674-4926/35/9/095011
****
X Y Shen, Z G Wang. Fully integrated circuit chip of microelectronic neural bridge[J]. J. Semicond., 2014, 35(9): 095011. doi: 10.1088/1674-4926/35/9/095011.
|
Fully integrated circuit chip of microelectronic neural bridge
DOI: 10.1088/1674-4926/35/9/095011
More Information
-
Abstract
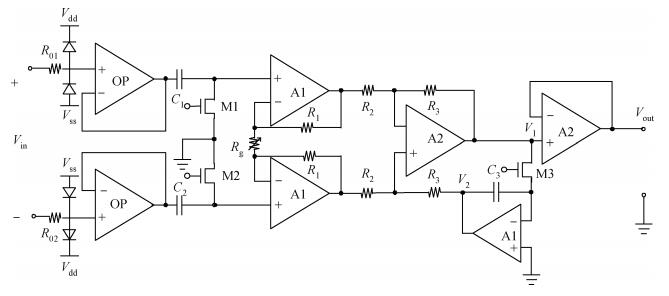
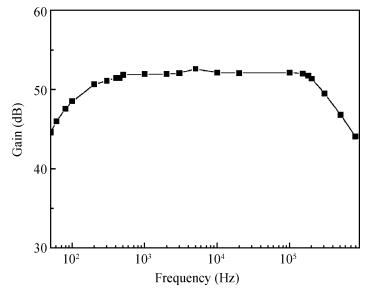
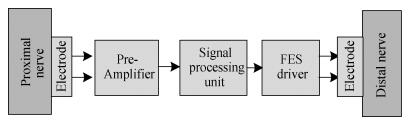
Nerve tracts interruption is one of the major reasons for dysfunction after spiral cord injury. The microelectronic neural bridge is a method to restore function of interrupted neural pathways, by making use of microelectronic chips to bypass the injured nerve tracts. A low-power fully integrated microelectronic neural bridge chip is designed, using CSMC 0.5-μm CMOS technology. The structure and the key points in the circuit design will be introduced in detail. In order to meet the requirement for implantation, the circuit was modified to avoid the use of off-chip components, and fully monolithic integration is achieved. The operating voltage of the circuit is ±2.5 V, and the chip area is 1.21×1.18 mm2. According to the characteristic of neural signal, the time-domain method is used in testing. The pass bandwidth of the microelectronic neural bridge system covers the whole frequency range of the neural signal, power consumption is 4.33 mW, and the gain is adjustable. The design goals are achieved. -
References
[1] Serruya M D, Kahana M J. Techniques and devices to restore cognition. Behavioral Brain Research, 2008, 192(2):149 doi: 10.1016/j.bbr.2008.04.007[2] Li H, Zhao W, Zhang Y. Micropower fully integrated CMOS readout interface for neural recording application. Microelectron Reliab, 2010, 50(2):273 doi: 10.1016/j.microrel.2009.09.013[3] Chao T, Askari S, de Leon R, et al. A system to integrate electrical stimulation with robotically controlled treadmill training to rehabilitate stepping after spinal cord injury. IEEE Trans Neural Syst Rehabil Eng, 2012, 20(5):730 doi: 10.1109/TNSRE.2012.2202292[4] Zhang J, Zhang X. Electrical stimulation of the dorsal cochlear nucleus induces hearing in rats. Brain Research, 2010, 1311:37 doi: 10.1016/j.brainres.2009.11.032[5] Tanriverdi T, Al-Jehani H, Poulin N, et al. Functional results of electrical cortical stimulation of the lower sensory strip. Journal of Clinical Neuroscience, 2009, 16(9):1188 doi: 10.1016/j.jocn.2008.11.010[6] Wang Z G, Lü X Y, Gu X S. Study of detecting, processing and rebuilding of central neural signals by using microelectronics. J Commun Comput, 2004:77[7] Huang Z, Wang Z, Lu X, et al. Design and experiment of a neural signal detection using a FES driving system. Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2010:1523 http://yadda.icm.edu.pl/yadda/element/bwmeta1.element.ieee-000005626834[8] Shen X, Wang Z, Lu X, et al. Experimental research on rebuilding the motive functions of a controlled spinal toad using MENB. High Technol Lett, 2011, 21(5):530[9] Shen Xiaoyan, Wang Zhigong, Lu Xiaoying, et al. Microelectronic neural bridge for signal regeneration and function rebuilding over two separate nerves. Journal of Semiconductors, 2011, 32(6):065011 doi: 10.1088/1674-4926/32/6/065011[10] Wang Z G, Lü X Y, Gu X S. Research of central nerve signal recording, processing and regeneration with microelectronics devices. 14th Conference on Neural Networks of China, Anhui, 2004 -
Proportional views





 DownLoad:
DownLoad: