| Citation: |
Yanlei Li, Yuling Liu, Chenwei Wang, Yue Li. Synergetic effect of chelating agent and nonionic surfactant for benzotriazoleremoval on post Cu-CMP cleaning[J]. Journal of Semiconductors, 2016, 37(8): 086001. doi: 10.1088/1674-4926/37/8/086001
****
Y L Li, Y L Liu, C W Wang, Y Li. Synergetic effect of chelating agent and nonionic surfactant for benzotriazoleremoval on post Cu-CMP cleaning[J]. J. Semicond., 2016, 37(8): 086001. doi: 10.1088/1674-4926/37/8/086001.
|
Synergetic effect of chelating agent and nonionic surfactant for benzotriazoleremoval on post Cu-CMP cleaning
DOI: 10.1088/1674-4926/37/8/086001
More Information
-
Abstract
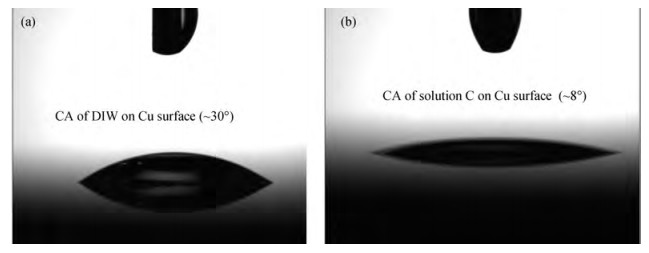
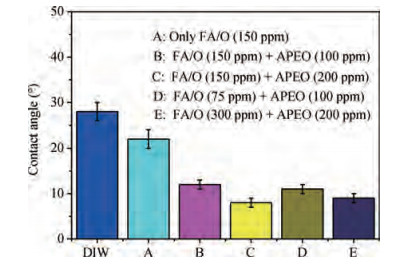
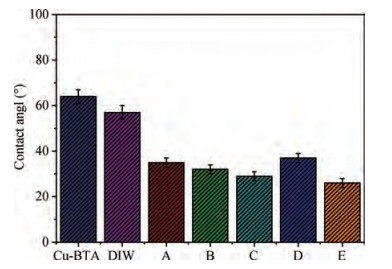
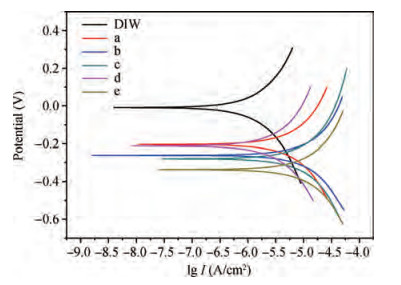
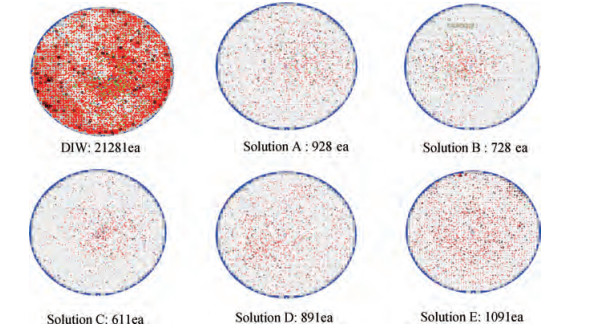
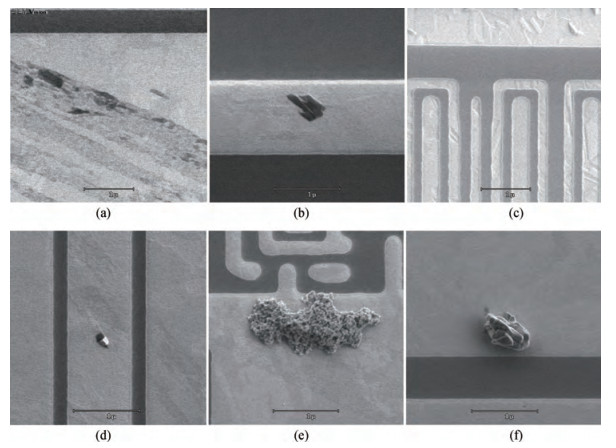
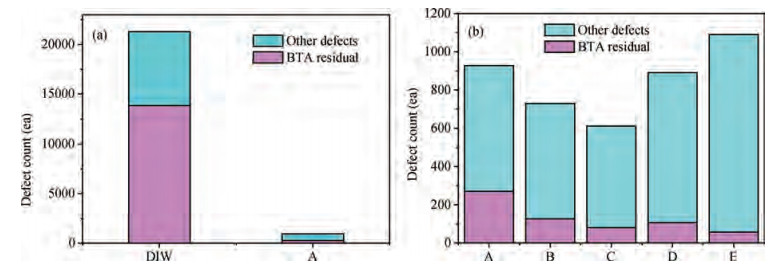
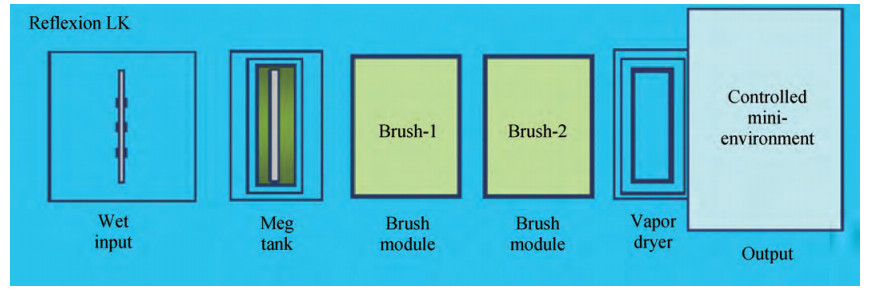
The cleaning of copper interconnects after chemical mechanical planarization (CMP) process is a critical step in integrated circuits (ICs) fabrication. Benzotriazole (BTA), which is used as corrosion inhibitor in the copper CMP slurry, is the primary source for the formation of organic contaminants. The presence of BTA can degrade the electrical properties and reliability of ICs which needs to be removed by using an effective cleaning solution. In this paper, an alkaline cleaning solution was proposed. The alkaline cleaning solution studied in this work consists of a chelating agent and a nonionic surfactant. The removal of BTA was characterized by contact angle measurements and potentiodynamic polarization studies. The cleaning properties of the proposed cleaning solution on a 300 mm copper patterned wafer were also quantified, total defect counts after cleaning was studied, scanning electron microscopy (SEM) review was used to identify types of BTA to confirm the ability of cleaning solution for BTA removal. All the results reveal that the chelating agent can effectively remove the BTA residual, nonionic surfactant can further improve the performance. -
References
[1] DeNardis D, Rosales-Yeomans D, Borucki L, et al. A three-step copper chemical mechanical planarization model including the dissolution effects of a commercial slurry. Thin Solid Films, 2010, 518(14): 3910 doi: 10.1016/j.tsf.2009.12.088[2] Chen Y H, Tsai T H, Yen S C. Acetic acid and phosphoric acid adding to improve tantalum chemical mechanical polishing in hydrogen peroxide-based slurry. Microelectron Eng, 2010, 87(2): 174 doi: 10.1016/j.mee.2009.07.009[3] Pandija S, Roy D, Babu S V. Achievement of high planarization efficiency in CMP of copper at a reduced down pressure. Microelectron Eng, 2009, 86(3): 367 doi: 10.1016/j.mee.2008.11.047[4] Wang J, Haerle A G. Chemical mechanical planarization of copper using transition alumina nanoparticles. Thin Solid Films, 2008, 516(21): 7648 doi: 10.1016/j.tsf.2008.06.029[5] Gelman D, Starosvetsky D, Ein-Eli Y. Copper corrosion mitigation by binary inhibitor compositions of potassium sorbate and benzotriazole. Corrosion Science, 2014, 82: 271 doi: 10.1016/j.corsci.2014.01.028[6] Li J, Liu Y, Wang T, et al. Electrochemical investigation of copper passivation kinetics and its application to low-pressure CMP modeling. Appl Surf Sci, 2013, 265: 764 doi: 10.1016/j.apsusc.2012.11.106[7] Kang M C, Kim Y J, Koo H C, et al. Local corrosion of the oxide passivation layer during Cu chemical mechanical polishing. Electrochemical and Solid-State Letters, 2009, 12(12): H433 doi: 10.1149/1.3236391[8] Lee H, Joo S, Jeong H. Mechanical effect of colloidal silica in copper chemical mechanical planarization. Journal of Materials Processing Technology, 2009, 209(20): 6134 doi: 10.1016/j.jmatprotec.2009.05.027[9] Oh Y J, Park G S, Chung C H. Planarization of copper layer for damascene interconnection by electrochemical polishing in alkali-based solution. Journal of the Electrochemical Society, 2006, 153(7): G617 doi: 10.1149/1.2200288[10] Nagar M, Vaes J, Ein-Eli Y. Potassium sorbate as an inhibitor in copper chemical mechanical planarization slurries. Part II: Effects of sorbate on chemical mechanical planarization performance. Electrochimica Acta, 2010, 55(8): 2810 doi: 10.1016/j.electacta.2009.10.086[11] Zhang Jin, Liu Yuling, Yan Chenqi, et al. Defectivity control of aluminum chemical mechanical planarization in replacement metal gate process of MOSFET. Journal of Semiconductors, 2016, 37(4): 046001 doi: 10.1088/1674-4926/37/4/046001[12] Feng Cuiyue, Liu Yuling, Sun Ming, et al. Investigation of aluminum gate CMP in a novel alkaline solution. Journal of Semiconductors, 2016, 37(1): 016002 doi: 10.1088/1674-4926/37/1/016002[13] Hu Yi, Li Yan, Liu Yuling, et al. The optimization of FA/O barrier slurry with respect to removal rate selectivity on patterned Cu wafers. Journal of Semiconductors, 2016, 37(2): 026003 doi: 10.1088/1674-4926/37/2/026003[14] Hong Jiao, Liu Yuling, Zhang Baoguo, et al. A new kind of chelating agent with low pH value applied in the TSV CMP slurry. Journal of Semiconductors, 2015, 36(12): 126001 doi: 10.1088/1674-4926/36/12/126001[15] Kim H J, Bohra G, Yang H, et al. Study of the cross contamination effect on post CMP in situ cleaning process. Microelectron Eng, 2015, 136: 36 doi: 10.1016/j.mee.2015.03.033[16] Tseng W T, Rill E, Backes B, et al. Post Cu CMP cleaning of polyurethane pad debris. ECS Journal of Solid State Science and Technology, 2014, 3(1): N3023 http://cn.bing.com/academic/profile?id=2043069596&encoded=0&v=paper_preview&mkt=zh-cn[17] Finšgar M, Milošev I. Inhibition of copper corrosion by 1, 2, 3-benzotriazole: a review. Corrosion Science, 2010, 52(9): 2737 doi: 10.1016/j.corsci.2010.05.002[18] Kim H C, Kim M J, Lim T, et al. Effects of nitrogen atoms of benzotriazole and its derivatives on the properties of electrodeposited Cu films. Thin Solid Films, 2014, 550: 421 doi: 10.1016/j.tsf.2013.10.124[19] Wang Y, Xiong X, Li G, et al. Ablation behavior of HfC protective coatings for carbon/carbon composites in an oxyacetylene combustion flame. Corrosion Science, 2012, 65: 549 doi: 10.1016/j.corsci.2012.08.064[20] Mezzi A, Angelini E, De Caro T, et al. Investigation of the benzotriazole inhibition mechanism of bronze disease. Surface and Interface Analysis, 2012, 44(8): 968 doi: 10.1002/sia.4841[21] Goswami A, Koskey S, Mukherjee T, et al. Study of pyrazole as copper corrosion inhibitor in Alkaline post chemical mechanical polishing cleaning solution. ECS Journal of Solid State Science and Technology, 2014, 3(10): P293 doi: 10.1149/2.0011410jss[22] Manivannan R, Cho B J, Hailin X, et al. Characterization of non-amine-based post-copper chemical mechanical planarization cleaning solution. Microelectron Eng, 2014, 122: 33 doi: 10.1016/j.mee.2014.02.034[23] Venkatesh R P, Kwon T Y, Prasad Y N, et al. Characterization of TMAH based cleaning solution for post Cu-CMP application. Microelectron Eng, 2013, 102: 74 doi: 10.1016/j.mee.2012.04.006[24] Lin J Y, West A C, Wan C C, et al. Evaluation of post-Cu CMP cleaning of organic residues using microfluidic device. Electrochemistry Communications, 2008, 10(5): 677 doi: 10.1016/j.elecom.2008.02.005[25] Ein-Eli Y, Starosvetsky D. Review on copper chemicalmechanical polishing (CMP) and post-CMP cleaning in ultra large system integrated (ULSI)-an electrochemical perspective. Electrochimica Acta, 2007, 52(5): 1825 doi: 10.1016/j.electacta.2006.07.039[26] Miao Y, Wang S, Wang C, et al. Effect of chelating agent on benzotriazole removal during post copper chemical mechanical polishing cleaning. Microelectron Eng, 2014, 130: 18 doi: 10.1016/j.mee.2014.08.012[27] Sun Mingbin, Gao Baohong, Wang Chenwei, et al. Non-ionic surfactant on particles removal in post-CMP cleaning. Journal of Semiconductors, 2015, 36(2): 026002 doi: 10.1088/1674-4926/36/2/026002 -
Proportional views





 DownLoad:
DownLoad: