| Citation: |
S. Ananthakumar, J. Ram Kumar, S. Moorthy Babu. Colloidal synthesis and characterization of Cu2ZnSnS4 nanoplates[J]. Journal of Semiconductors, 2017, 38(3): 033007. doi: 10.1088/1674-4926/38/3/033007
****
S Ananthakumar, J R Kumar, S M Babu. Colloidal synthesis and characterization of Cu2ZnSnS4 nanoplates[J]. J. Semicond., 2017, 38(3): 033007. doi: 10.1088/1674-4926/38/3/033007.
|
Colloidal synthesis and characterization of Cu2ZnSnS4 nanoplates
DOI: 10.1088/1674-4926/38/3/033007
More Information
-
Abstract
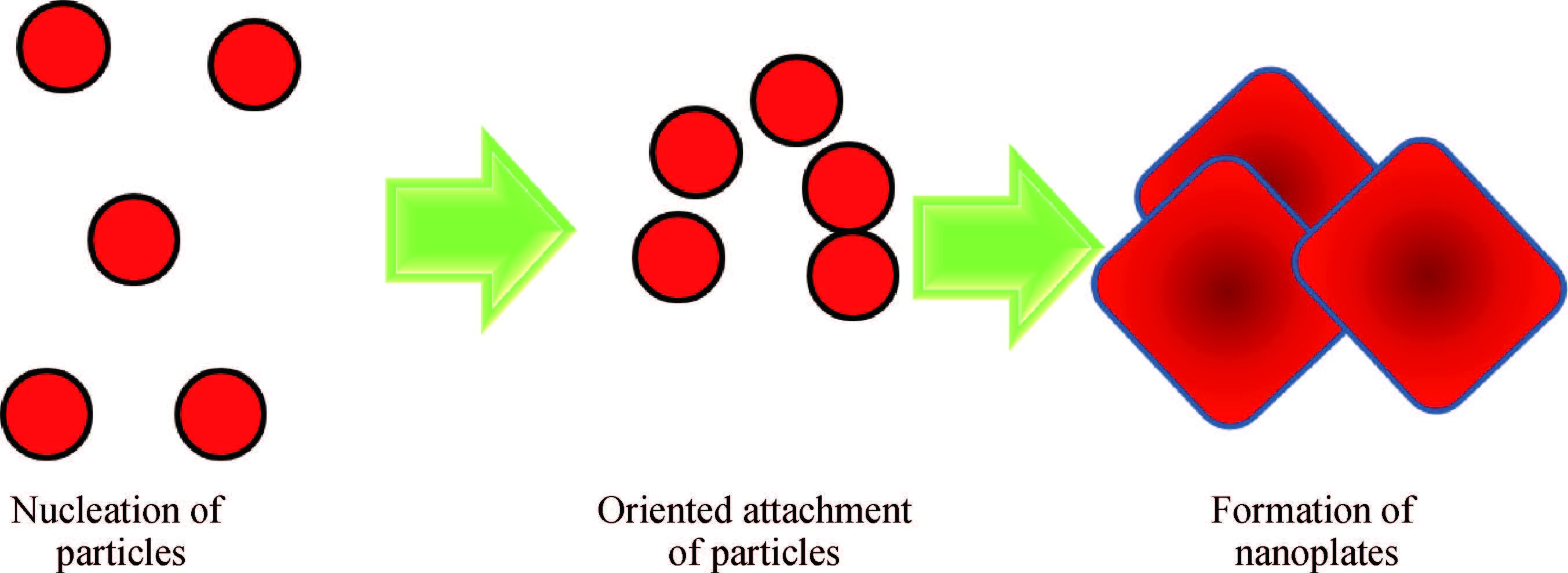
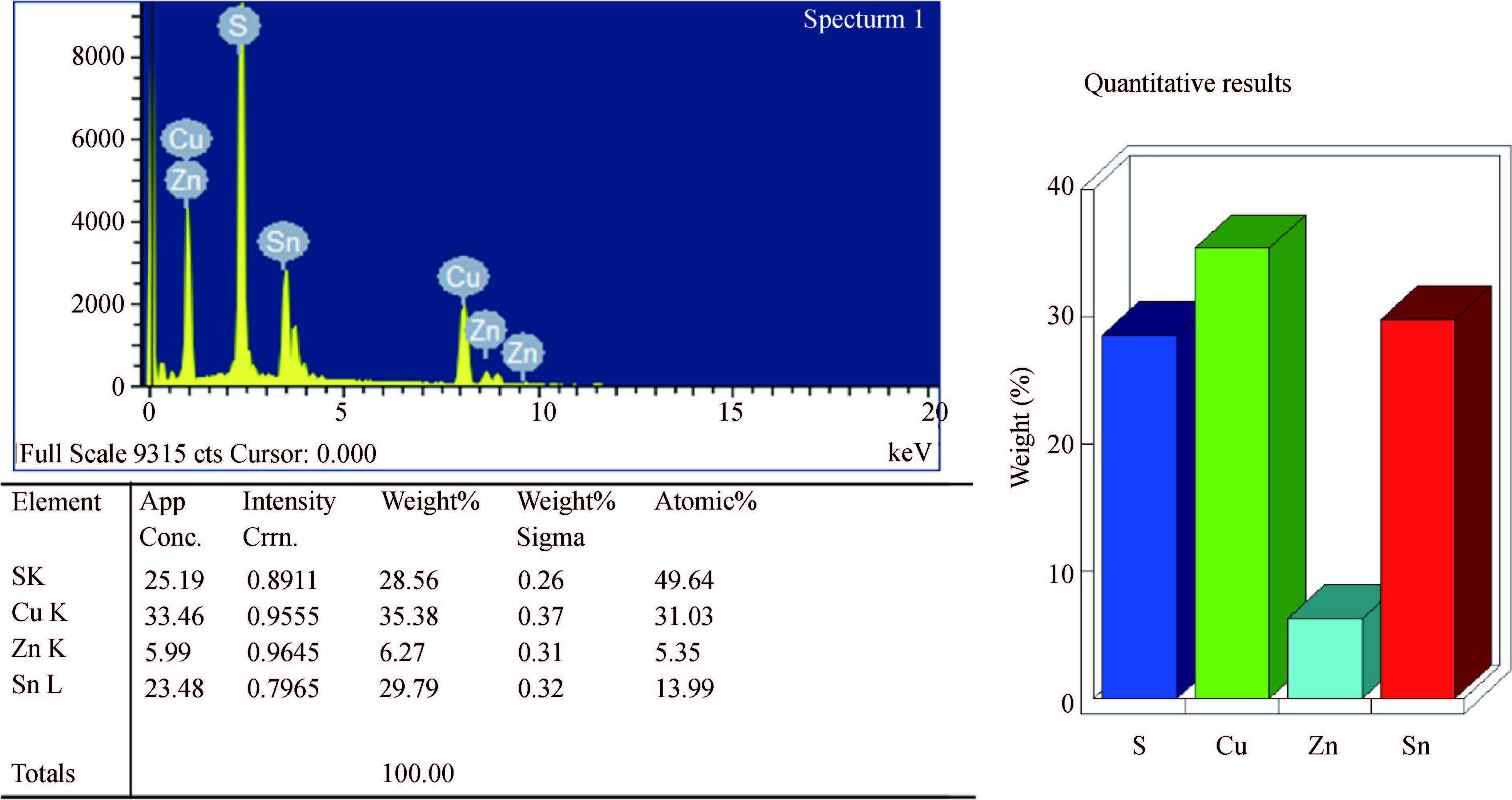
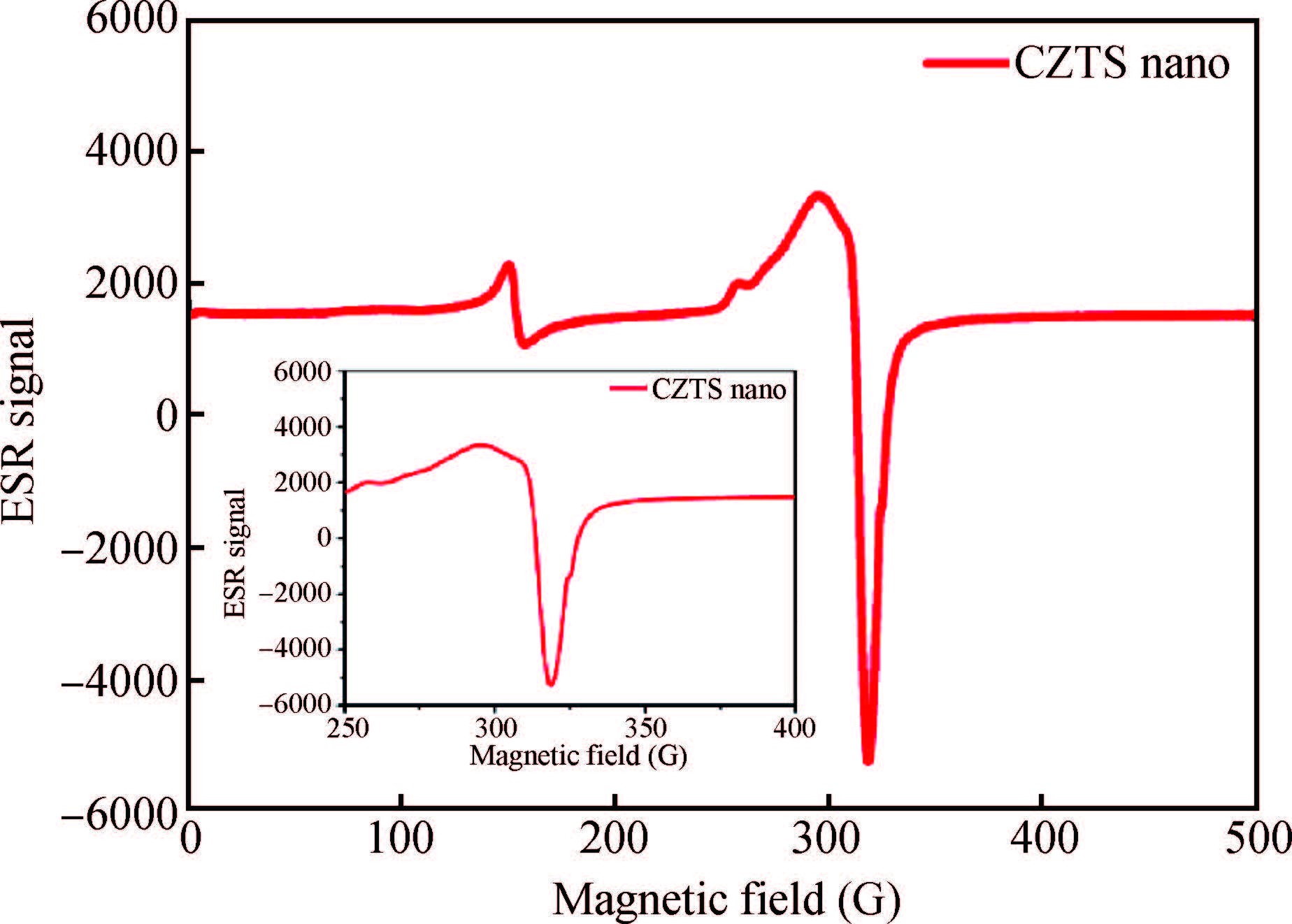
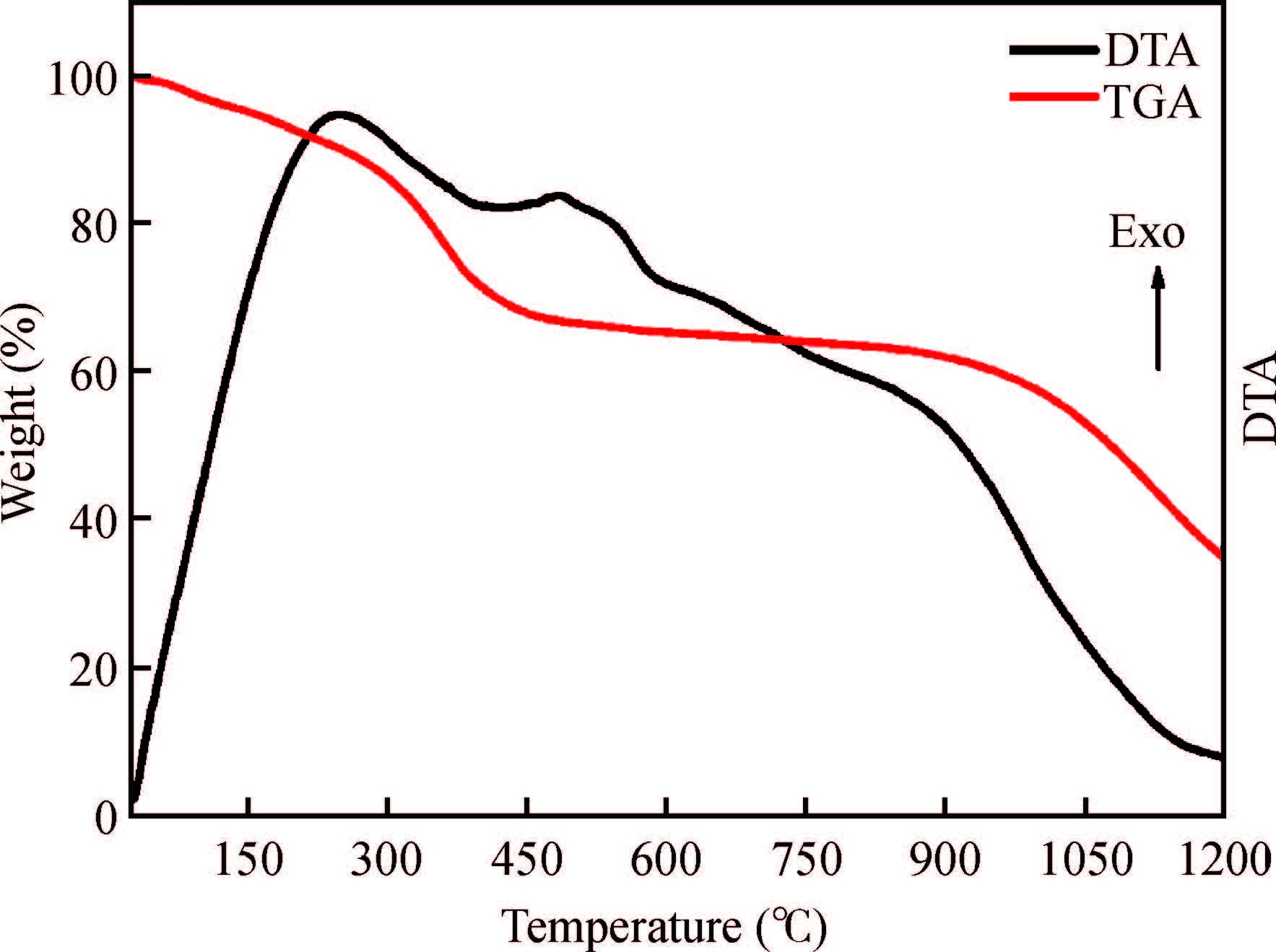
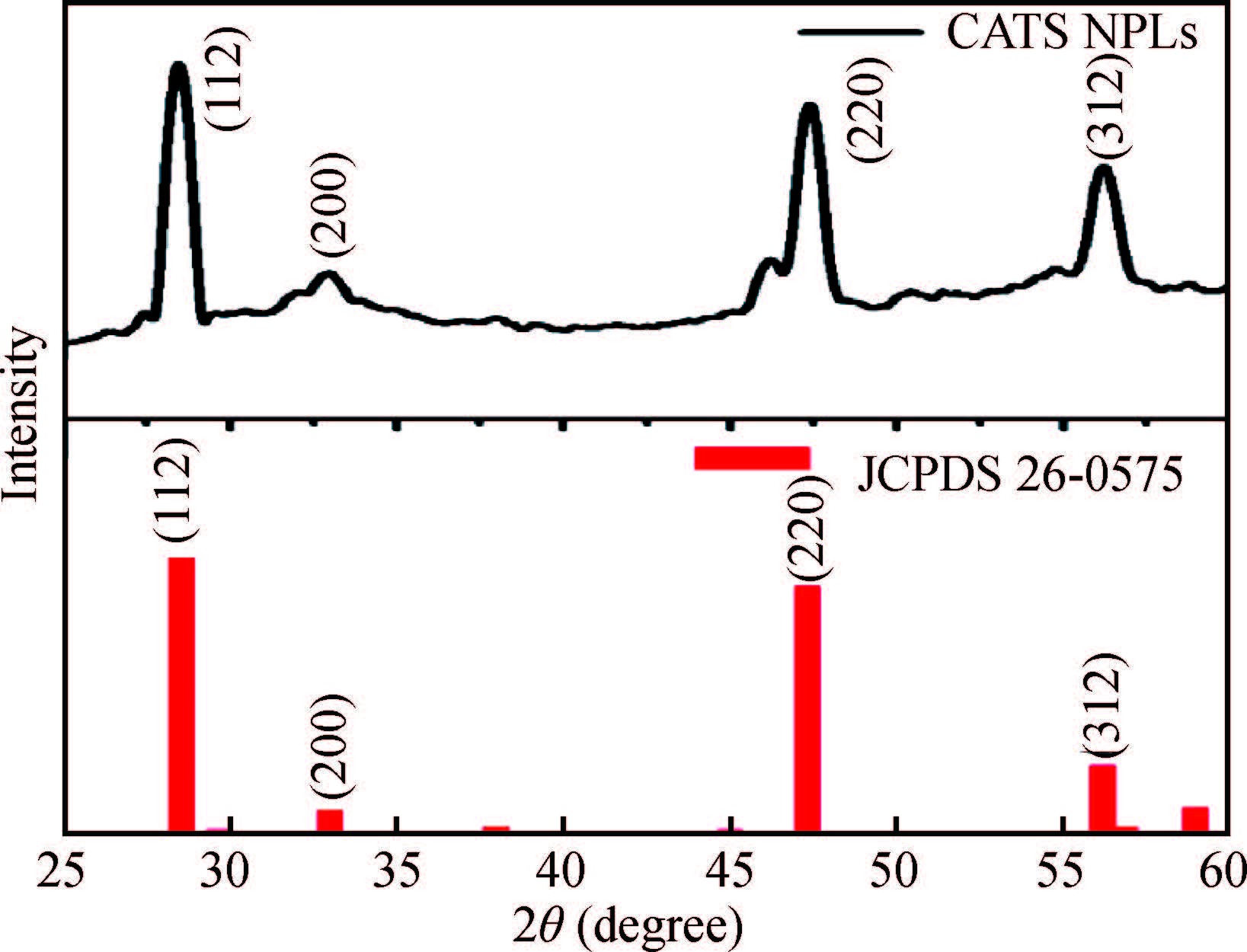
Synthesis of copper zinc tin sulphide (Cu2ZnSnS4) with nanoplate morphology was achieved through colloidal method using oleic acid as capping agent and solvent with 1-octadecene (1-ODE) at 240℃. X-ray diffraction (XRD) analysis shows that the synthesized nanoplates possessed pure kesterite phase. SEM analysis clearly shows the formation of nanoplates having the size of about 50-100 nm. Electron spin resonance (ESR) spectrum analysis of the prepared nanoplates shows that the valence state of copper (Ⅱ) which indicates the strong coupling with other metal ions. Thermo gravimetric/differential thermal analysis (TG/DTA) analysis shows the weight loss of sample at 450℃ predicting the loss of capping ligands on the surface of the nanoparticles. The possible mechanism for the conversion of nanoplate-like structures during synthesis was discussed. The results are discussed in detail.-
Keywords:
- colloidal method,
- solar cells,
- kesterites,
- nanoplates,
- oleic acid
-
References
[1] Abermmaan S. Non-vacuum processed next generation thin film photovoltaics:towards marketable efficiency and production of CZTS based solar cells. Sol Energy, 2013, 94:70 https://www.researchgate.net/publication/273807751_Non-vacuum_processed_next_generation_thin_film_photovoltaics_Towards_marketable_efficiency_and_production_of_CZTS_based_solar_cells[2] Ding S, Shengzhi X, Li Z, et al. Influence of selenium evaporation temperature on the structure of Cu2ZnSnSe4 thin film deposited by a co-evaporation process. J Semicond, 2015, 36(4):044009 doi: 10.1088/1674-4926/36/4/044009[3] Ding S, Yang G, Li Z, et al. Impact of Cu-rich growth on the Cu2ZnSnSe4 surface morphology and related solar cells behavior. J Semicond, 2016, 37(1):013004 doi: 10.1088/1674-4926/37/1/013004[4] Ramasamy K, Malik M A, O'Brien P. Routes to copper zinc tin sulfide Cu2ZnSnS4 a potential material for solar cells. Chem Commun, 2012, 48:5703 doi: 10.1039/c2cc30792h[5] Liu W C, Guo B L, Wu X S, et al. Facile hydrothermal synthesis of hydrotropic Cu2ZnSnS4 nanocrystal quantum dots:band-gap engineering and phonon confinement effect. J Mater Chem A, 2013, 1:3182 doi: 10.1039/c3ta00357d[6] Zhou J, You L, Li S, et al. Preparation and characterization of Cu2ZnSnS4 microparticles via a facile solution route. Mater Lett, 2012, 81:248 doi: 10.1016/j.matlet.2012.05.023[7] Jiang F, Shen H. Research on the photoresponse current and photosensitive properties of Cu2ZnSnS4 thin film prepared by sulfurization of a sputtered metal precursor. RSC Adv, 2013, 3:23474 doi: 10.1039/c3ra42333f[8] Liu F, Li Y, Zhang K, Wang B, et al. In situ growth of Cu2ZnSnS4 thin films by reactive magnetron co-sputtering. Sol Energy Mater Sol Cells, 2010, 94:2431 doi: 10.1016/j.solmat.2010.08.003[9] Katagiri H. Cu2ZnSnS4 thin film solar cells. Thin Solid Films, 2005, 480/481:426 doi: 10.1016/j.tsf.2004.11.024[10] Mali S S, Patil B M, Betty C A, et al. Novel synthesis of kesterite Cu2ZnSnS4 nanoflakes by successive ionic layer adsorption and reaction technique:characterization and application. Electrochem Acta, 2012, 66:216 doi: 10.1016/j.electacta.2012.01.079[11] Zhao Z Y, Zhao X. Electronic, optical and mechanical properties of Cu2ZnSnS4 with four crystal structures. J Semicond, 2015, 36(8):083004 doi: 10.1088/1674-4926/36/8/083004[12] Zhao Y, Zhou W H, Jiao J, et al. Aqueous synthesis and characterization of hydrophilic Cu2ZnSnS4 nanocrystals. Mater Lett, 2013, 96:174 doi: 10.1016/j.matlet.2013.01.059[13] Xie W, Jiang X, Zou C, Li D, et al. Synthesis of highly dispersed Cu2ZnSnS4 nanoparticles by solvothermal method for photovoltaic application. Physica E, 2012, 45:16 doi: 10.1016/j.physe.2012.05.022[14] Flynn B, Braly I, Glover P A, et al. Continuous flow mesofluidic synthesis of Cu2ZnSnS4 nanoparticle Inks. Mater Lett, 2013, 107:214 doi: 10.1016/j.matlet.2013.06.023[15] Zhou H, Hsu W C, Duan H S, et al. CZTS nanocrystals:a promising approach for next generation thin film photovoltaics. Energy Environ Sci, 2013, 6:2822 doi: 10.1039/c3ee41627e[16] Wang C L, Manthiram A. Low-cost CZTSSe solar cells fabricated with low band gap CZTSe nanocrystals, environmentally friendly binder, and non vacuum processes. ACS Sustainable Chem Eng, 2014, 2(4):561 doi: 10.1021/sc400465m[17] Todorov T K, Reuter K B, Mitzi D B. High-efficiency solar cell with earth-abundant liquid processed absorber. Adv Mater, 2010, 22(20):E156 doi: 10.1002/adma.200904155[18] Wozny S, Wang K, Zhou W. Cu2ZnSnS4 nanoplate arrays synthesized by pulsed laser deposition with high catalytic activity as counter electrodes for dye-sensitized solar cell applications. J Mater Chem A, 2013, 1:15517 doi: 10.1039/c3ta13358c[19] Chang J, Waclawik E R. Controlled synthesis of CuInS2, Cu2SnS3 and Cu2ZnSnS4 nano-structures:insight into the universal phase-selectivity mechanism. Cryst Eng Comm, 2013, 15:5612 doi: 10.1039/c3ce40284c[20] Lee J, Lee S H, Hahn J S, et al. Effects of solvents on the synthesis of CuInSe2 nanoparticles for thin film solar cells. J Nanosci Nanotechnol, 2014, 14(12):9313 doi: 10.1166/jnn.2014.10146[21] Gong F, Tian S, Liu B, et al. Oleic acid assisted formation mechanism of CuInS2 nanocrystals with tunable structures. RSC Adv, 2014, 4:36875 doi: 10.1039/C4RA03957B[22] Freymeyer N J, Cunningham P D, Jones E C, et al. Influence of solvents reducing ability on copper sulfide crystal phase. Cryst Growth Des, 2013, 13(9):4059 doi: 10.1021/cg400895d[23] Li J, Bloemen M, Parisi J, et al. Role of copper sulfide seeds in the growth process of CuInS2 nanorods and networks. ACS Appl Mater Interfaces, 2014, 6(22):20535 doi: 10.1021/am5061454[24] Yu W W, Peng X. Formation of high-quality CdS and other Ⅱ-VI semiconductor nanocrystals in non-coordinating solvents:tunable reactivity of monomers. Angewd Chem, 2002, 41(13):2368 doi: 10.1002/(ISSN)1521-3773[25] Edler M, Rath T, Schenk A, et al. Copper zinc tin sulfide layers prepared from solution processable metal dithiocarbamate precursors. Mater Chem Phys, 2012, 136:582 doi: 10.1016/j.matchemphys.2012.07.030[26] Kameyama T, Osaki T, Okazaki K I, et al. Preparation and photoelectrochemical properties of densely immobilized Cu2ZnSnS4 nanoparticle films. J Mater Chem, 2010, 20:5319 doi: 10.1039/c0jm00454e[27] Tan J M R, Lee Y H, Pedireddy S, et al. Understanding the synthetic pathway of a single-phase quaternary semiconductor using surface-enhanced Raman scattering:a case of wurtzite Cu2ZnSnS4 nanoparticles. J Am Chem Soc, 2014, 136(18):6684 doi: 10.1021/ja501786s[28] McPhail M R, Weiss E A. Role of organosulfur compounds in the growth and final surface chemistry of PbS quantum dots. Chem Mater, 2014, 26:3377 doi: 10.1021/cm4040819[29] Zhang X, Liu Q, Meng L, et al. In-plane co assembly route to atomically thick inorganic-organic hybrid nanosheets. ACS Nano, 2013, 7(2):1682 doi: 10.1021/nn3056719[30] Acharya S, Dutta M, Sarkar S, et al. Synthesis of micrometer length indium sulfide nanosheets and study of their dopant induced photoresponse properties. Chem Mater, 2012, 24(10):1779 doi: 10.1021/cm3003063[31] Chang S H, Chiu B C, Gao T L, et al. Selective synthesis of copper gallium sulfide (CuGaS2/nanostructures of different sizes, crystal phases, and morphologies. Cryst Eng Comm, 2014, 16:3323 doi: 10.1039/c3ce42530d[32] Chory C, Zutz F, Witt F, et al. Synthesis and characterization of Cu2ZnSnS4. Phys Status Solidi C, 2010, 7(6):1486 doi: 10.1002/pssc.v7:6[33] Khan M A M, Kumar S, Alhoshan M, et al. Spray pyrolysed Cu2ZnSnS4 absorbing layer:a potential candidate for photovoltaic applications. Opt Laser Technol, 2013, 49:196 doi: 10.1016/j.optlastec.2012.12.012 -
Proportional views





 DownLoad:
DownLoad: