| Citation: |
Fengyu Xie, Jiacheng Gao, Ning Wang. Photoelectrochemical performance of La3+-doped TiO2[J]. Journal of Semiconductors, 2017, 38(7): 073002. doi: 10.1088/1674-4926/38/7/073002
****
F Y Xie, J C Gao, N Wang. Photoelectrochemical performance of La3+-doped TiO2[J]. J. Semicond., 2017, 38(7): 073002. doi: 10.1088/1674-4926/38/7/073002.
|
Photoelectrochemical performance of La3+-doped TiO2
DOI: 10.1088/1674-4926/38/7/073002
More Information
-
Abstract
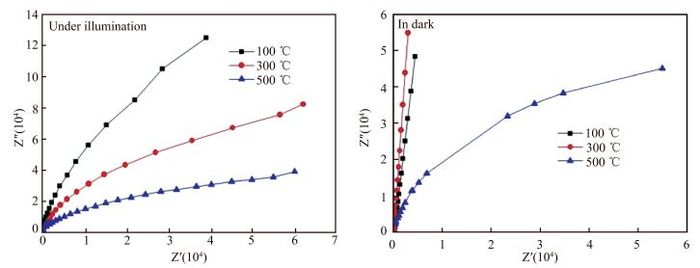
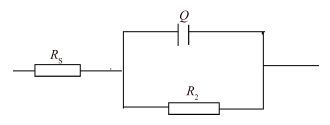
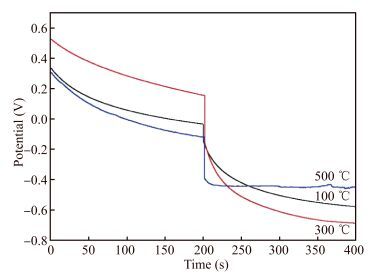
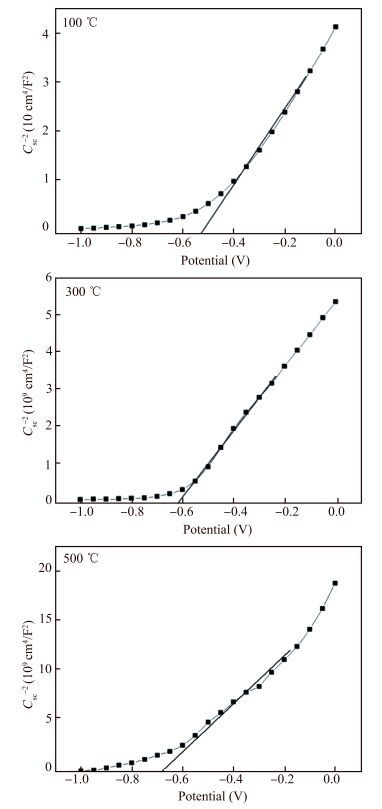
La-doped TiO2 thin films on titanium substrates were prepared by the sol-gel method with titanium tetrachloride as a precursor and La2O3 as a source of lanthanum. The heat-treatment temperature dependence of the photoelectrochemical performance of the La-doped TiO2 film in 0.2 mol/L Na2SO4 was investigated by the Mott-Schottky equation, electrochemical impedance spectroscopy, and the open-circuit potential test. The results from the Mott-Schottky curves show that the obtained films all were n-type semiconductors, and the film at 300 ℃ had the highest conduction band position and the widest space charge layer. The electrochemical impendence spectroscopy (EIS) tests of the 300 ℃ film decreased most during the change from illuminated to dark. The potential of the La-TiO2 thin film electrode was the lowest after the 300 ℃ heat treatment. The open-circuit potential indicated that the photoelectrical performance of the La-TiO2 films was enhanced with the addition of the La element and the largest decline (837.8 mV) in the electrode potential was achieved with the 300 ℃ heat treatment.-
Keywords:
- TiO2,
- La3+-doped,
- heat treatment,
- photoelectrochemical performance
-
References
[1] Chen C, Ye M, Lv M, et al. Ultralong rutile TiO2 nanorod arrays with large surface area for CdS/CdSe quantum dot-sensitized solar cells. Electrochim Acta, 2014, 121(3): 175 http://or.nsfc.gov.cn/bitstream/00001903-5/78757/1/1000007434458.pdf[2] He Z Q, Cai Q L, Fang H Y, et al. Photocatalytic activity of TiO2 containing anatase nanoparticles and rutile nanoflower structure consisting of nanorods. J Environ Sci, 2013, 25(12): 2460 doi: 10.1016/S1001-0742(12)60318-0[3] Luo Q, Cai Qi Z, Li X W, et al. Preparation and characterization of ZrO2/TiO2 composite photocatalytic film by micro-arc oxidation. Trans Nonferrous Met Soc China, 2013, 23(10): 2945 doi: 10.1016/S1003-6326(13)62818-6[4] Boschloo G K, Goossens A, Schoonman J. Photoelectrochemical study of thin anatase TiO2 films prepared by metallorganic chemical vapor deposition. Chemlnform, 1997, 28(32): 1311 https://www.osti.gov/scitech/biblio/509388[5] Li J, Lin C J, Li, J T, et al. A photoelectrochemical study of CdS modified TiO2 nanotube arrays as photoanodes for cathodic protection of stainless steel. Thin Solid Films, 2011, 519(16): 5494 doi: 10.1016/j.tsf.2011.03.116[6] Zhang J, Du R G, Lin Z Q, et al. Highly efficient CdSe/CdS co-sensitized TiO2 nanotube films for photocathodic protection of stainless steel. Electrochim Acta, 2012, 83: 59 doi: 10.1016/j.electacta.2012.07.120[7] Li Y, Xu C, Feng Z D. Characteristics and anticorrosion performance of Fe-doped TiO2 films by liquid phase deposition method. Appl Surf Sci, 2014, 314: 392 doi: 10.1016/j.apsusc.2014.07.042[8] Sun M, Chen Z, Yu J. Highly efficient visible light induced photoelectro-chemical anticorrosion for 304 SS by Ni-doped TiO2. Electrochim Acta, 2013, 109: 13 doi: 10.1016/j.electacta.2013.07.121[9] Li S, Wang Q, Chen T, et al. Study on cerium-doped nano-TiO2 coatings for corrosion protection of 316 L stainless steel. Nanoscale Res Lett, 2012, 7(1): 1 doi: 10.1186/1556-276X-7-1[10] Li J, Lin C J, Lai Y K, et al. Photogenerated cathodic protection of flower-like, nanostructured, N-doped TiO2 film on stainless steel. Surf Coat Technol, 2010, 205(2): 557 doi: 10.1016/j.surfcoat.2010.07.030[11] Rodriguez-Talavera R, Vargas S, Arroyo-Murillo R, et al. Modification of the phase transition temperatures in titania doped with various cations. J Mater Res, 1997, 12(02): 439 doi: 10.1557/JMR.1997.0065[12] Wang Y, Cheng H, Zhang L, et al. The preparation, characterization, photo-electrochemical and photocatalytic properties of lanthanide metal-ion-doped TiO2 nanoparticles. J Mol Catal A, 2000, 151(1): 205[13] Yang S, Huang Y, Huang C, et al. Enhanced energy conversion efficiency of the Sr2+-modified nanoporous TiO2 electrode sensitized with a ruthenium complex. Chem Mater, 2002, 14(4): 1500 doi: 10.1021/cm010609e[14] Dewald J F. The charge distribution at the zinc oxide-electrolyte interface. J Phys Chem Solids, 1960, 14: 155 doi: 10.1016/0022-3697(60)90223-7[15] Büchler M, Schmuki P, Böhni H. A light reflectance technique for thickness measurements of passive films. Electrochim Acta, 1998, 43(5): 635 http://www.sciencedirect.com/science/article/pii/S0013468697001229?via%3Dihub[16] Leng W, Zhao Z, Cheng S, et al. A study of Titanium oxide film electrodes prepared by direct thermal oxidation preparation, structure and electrochemical properties. Chin J Chem Phys, 2001, 39(3): 7172[17] Sikora J, Sikora E, Macdonald D D. Electronic structure of the passive film on tungsten. Electrochim Acta, 2000, 45(12): 1875 doi: 10.1016/S0013-4686(99)00407-7[18] Schmuki P, Bohni H. Illumination effects on the stability of the passive on iron. Electrochim Acta, 1995, 40(6): 775 doi: 10.1016/0013-4686(94)00341-W[19] Morison S R. Electrochemistry at semiconductor and oxidized metal electrodes. Plenum Press, 1980[20] Macdonald D D, Ismail K M, Sikora E. Characterization of passive state on zinc. J Environ Sci, 1998, 145(9): 3141 http://jes.ecsdl.org/content/145/9/3141.abstract?cited-by=yesl145/9/3141r145/9/3141[21] Sang L X, Zhang Z Y, Bai G M, et al. A photoelectrochemical investigation of the hydrogen-evolving doped TiO2, nanotube arrays electrode. Fuel Energy Abstracts, 2012, 37(1): 854[22] Dong W H, Kim J, Park T J, et al. Mg-doped WO3 as a novel photocatalyst for visible light-induced water splitting. Catal Lett, 2002, 80(1): 53[23] Wang B H, Wang D J, Li T J. Studies on photoelectrochemical properties of porous silicon. Chem Res Chin Univ, 1997(4): 621 http://en.cnki.com.cn/Article_en/CJFDTOTAL-GDXH199704028.htm[24] Naderi R, Attar M M. The inhibitive performance of polyphosphate-based anticorrosion pigments using electrochemical techniques. Dyes Pigments, 2009, 80(3): 349 doi: 10.1016/j.dyepig.2008.08.002 -
Proportional views





 DownLoad:
DownLoad: