| Citation: |
Hammou Gherras, Ahmed Yahiaoui, Aicha Hachemaoui, Abdelkader Belfedal, Abdelkader Dehbi, Abdel-Hamid I. Mourad. Synthesis and characterization of poly (2,5-diyl pyrrole-2-pyrrolyl methine) semiconductor copolymer[J]. Journal of Semiconductors, 2018, 39(10): 102001. doi: 10.1088/1674-4926/39/10/102001
****
H Gherras, A Yahiaoui, A Hachemaoui, A Belfedal, A Dehbi, A H I Mourad, Synthesis and characterization of poly (2,5-diyl pyrrole-2-pyrrolyl methine) semiconductor copolymer[J]. J. Semicond., 2018, 39(10): 102001. doi: 10.1088/1674-4926/39/10/102001.
|
Synthesis and characterization of poly (2,5-diyl pyrrole-2-pyrrolyl methine) semiconductor copolymer
DOI: 10.1088/1674-4926/39/10/102001
More Information
-
Abstract
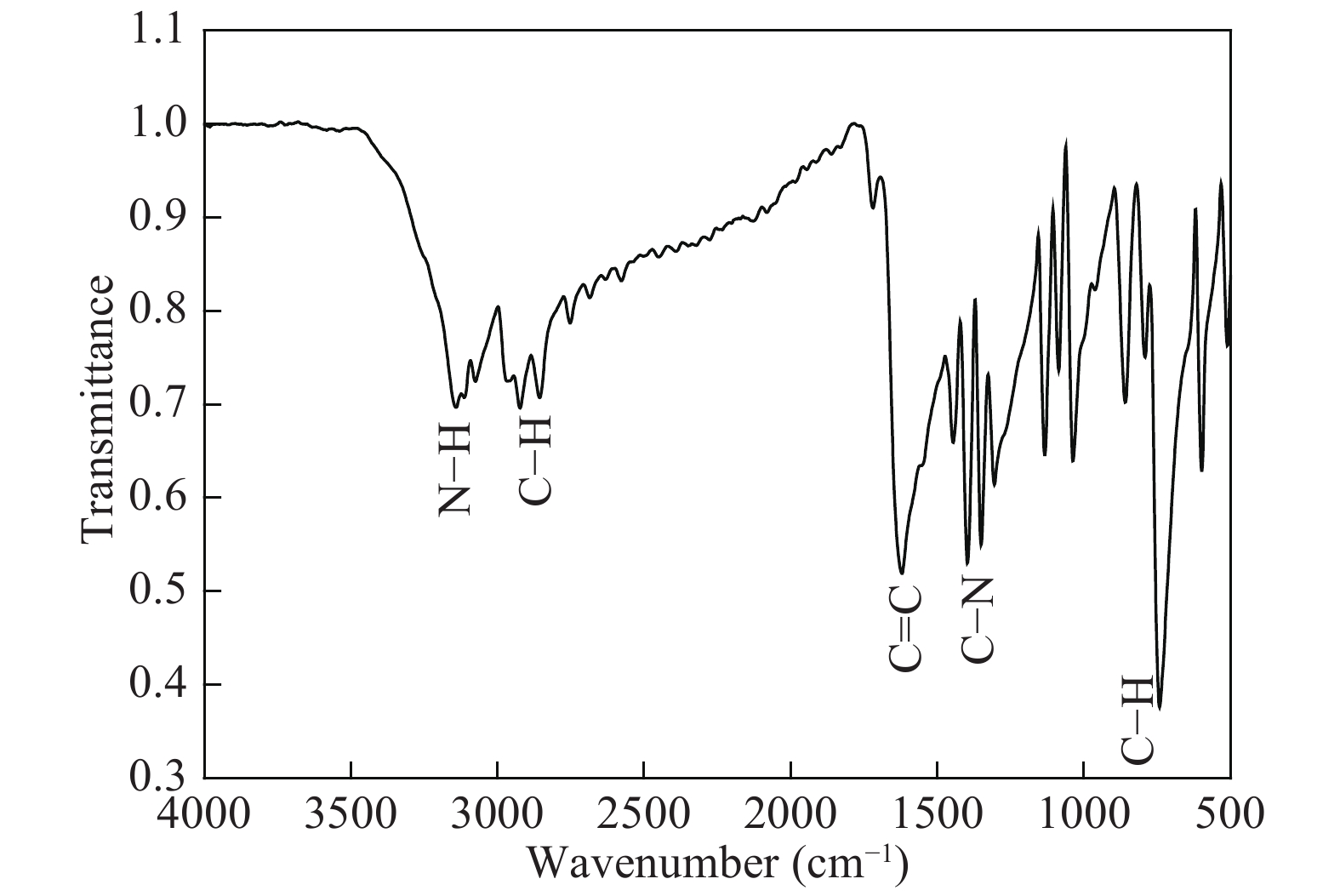
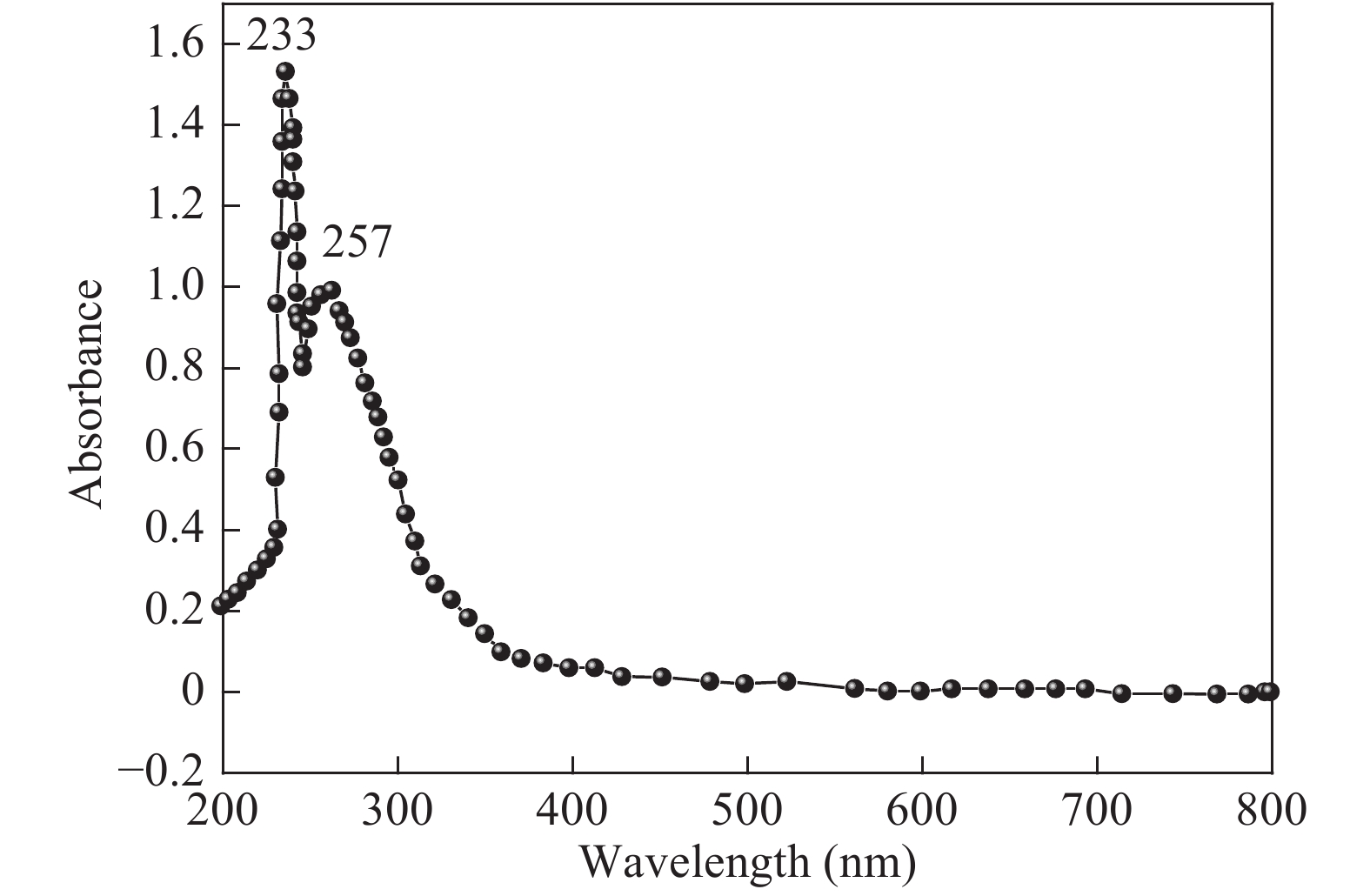
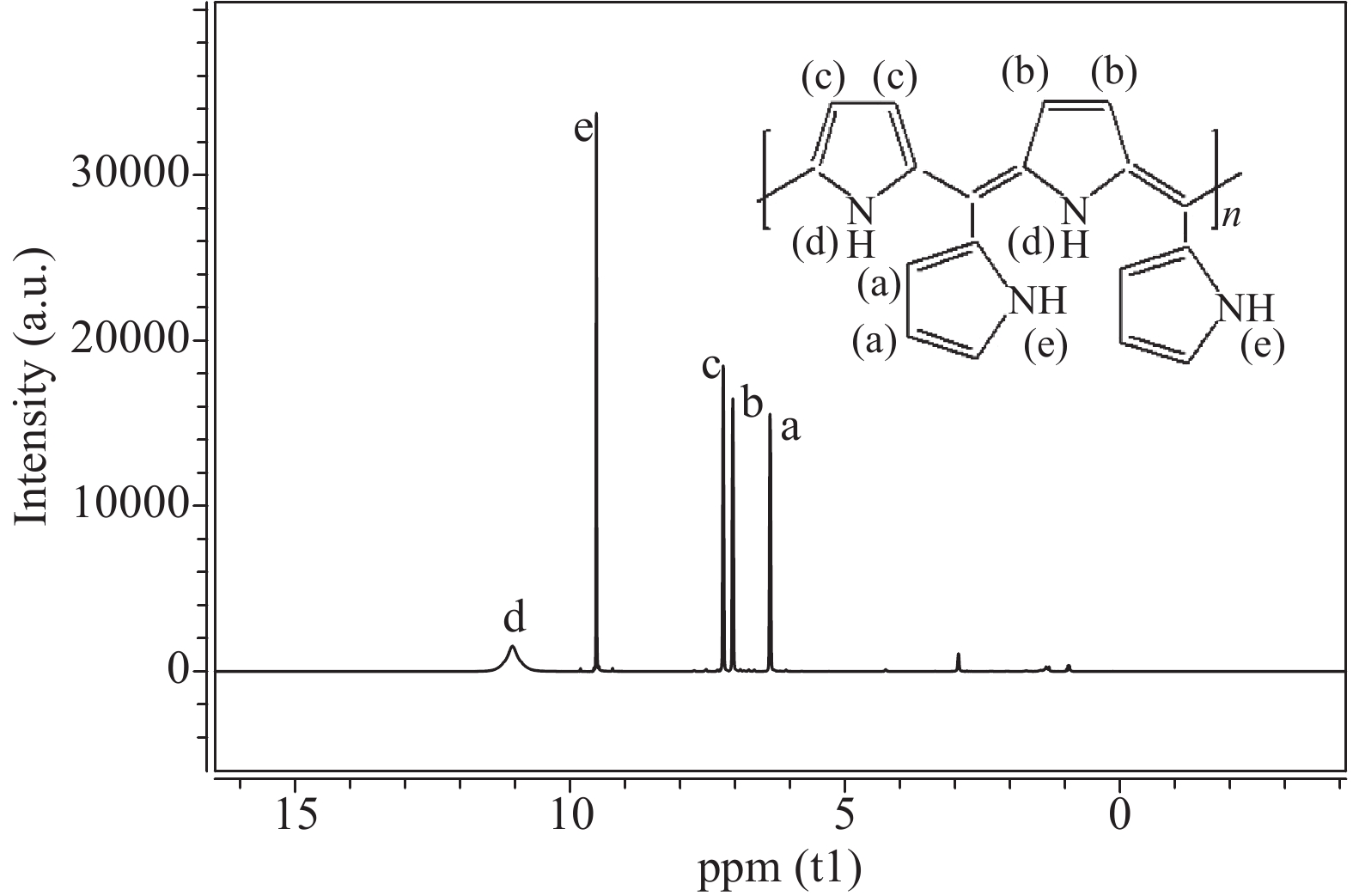
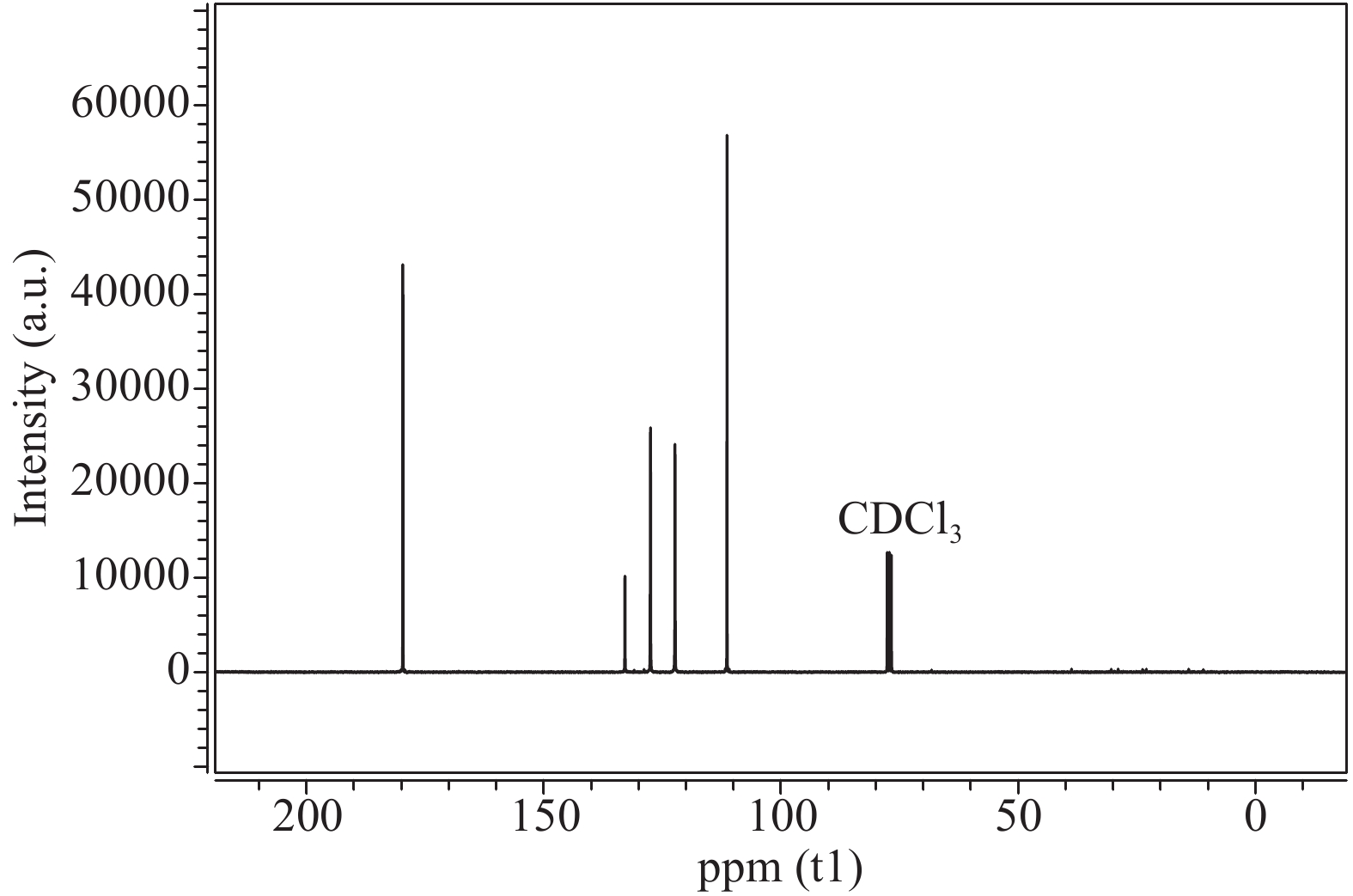
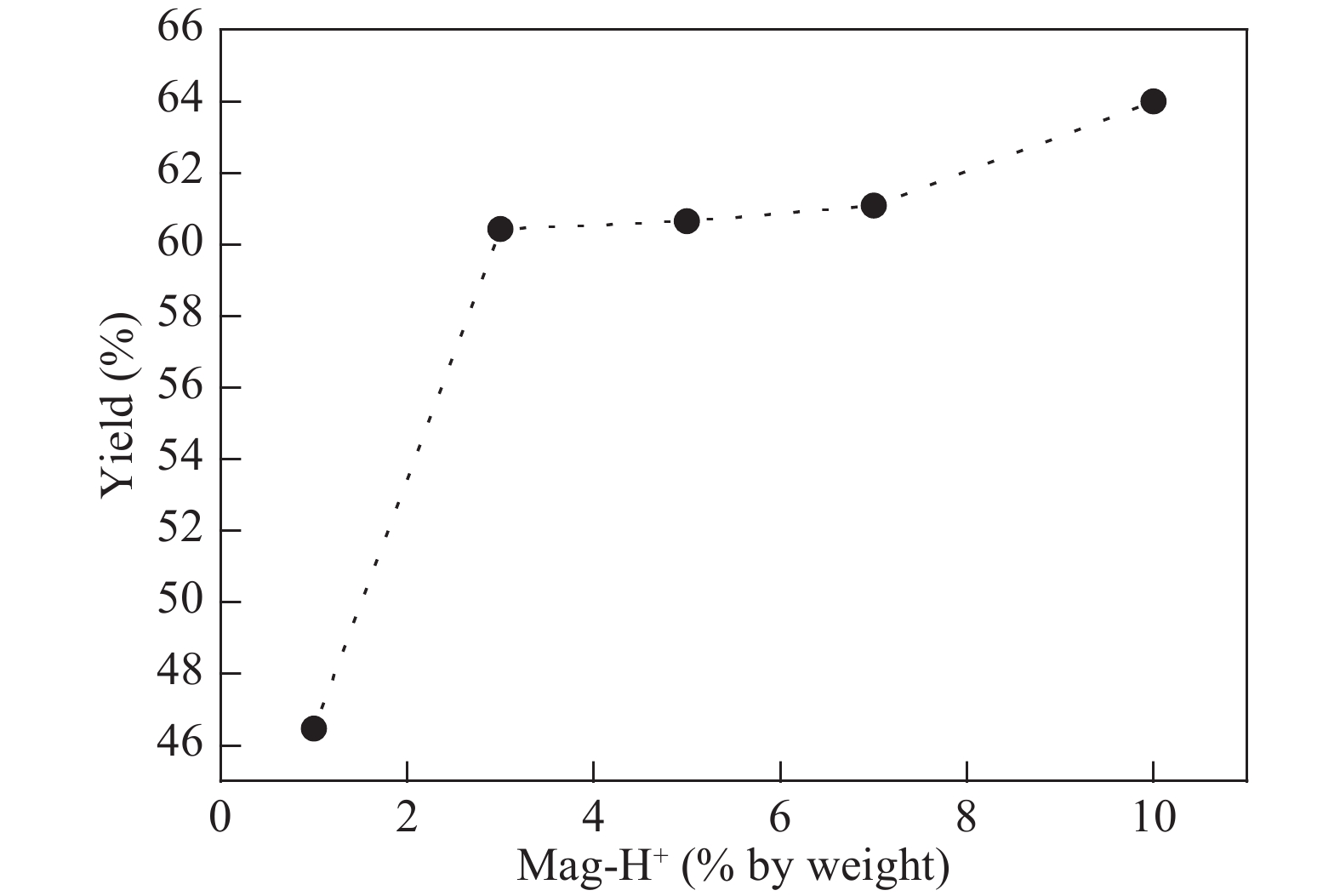
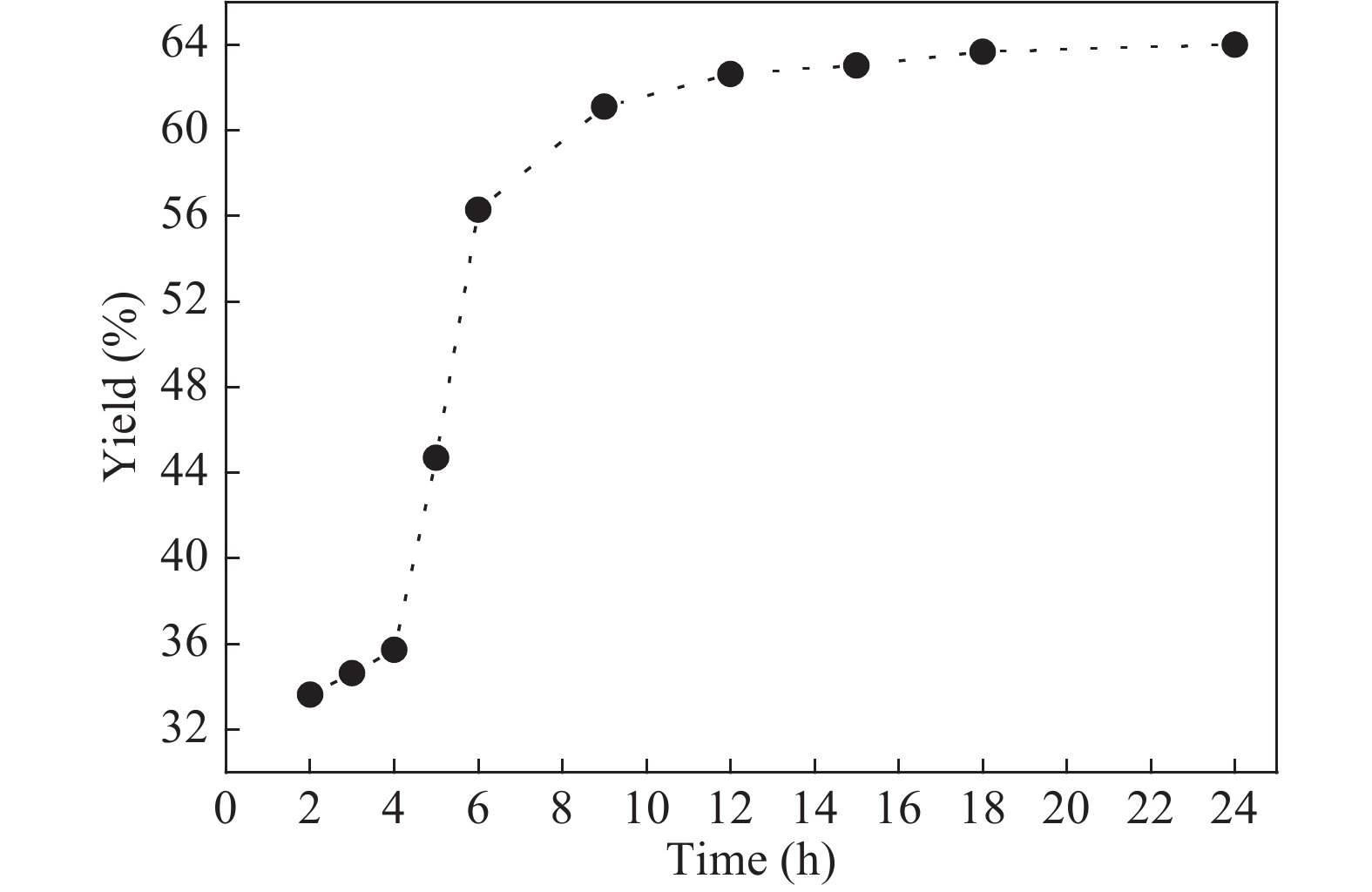
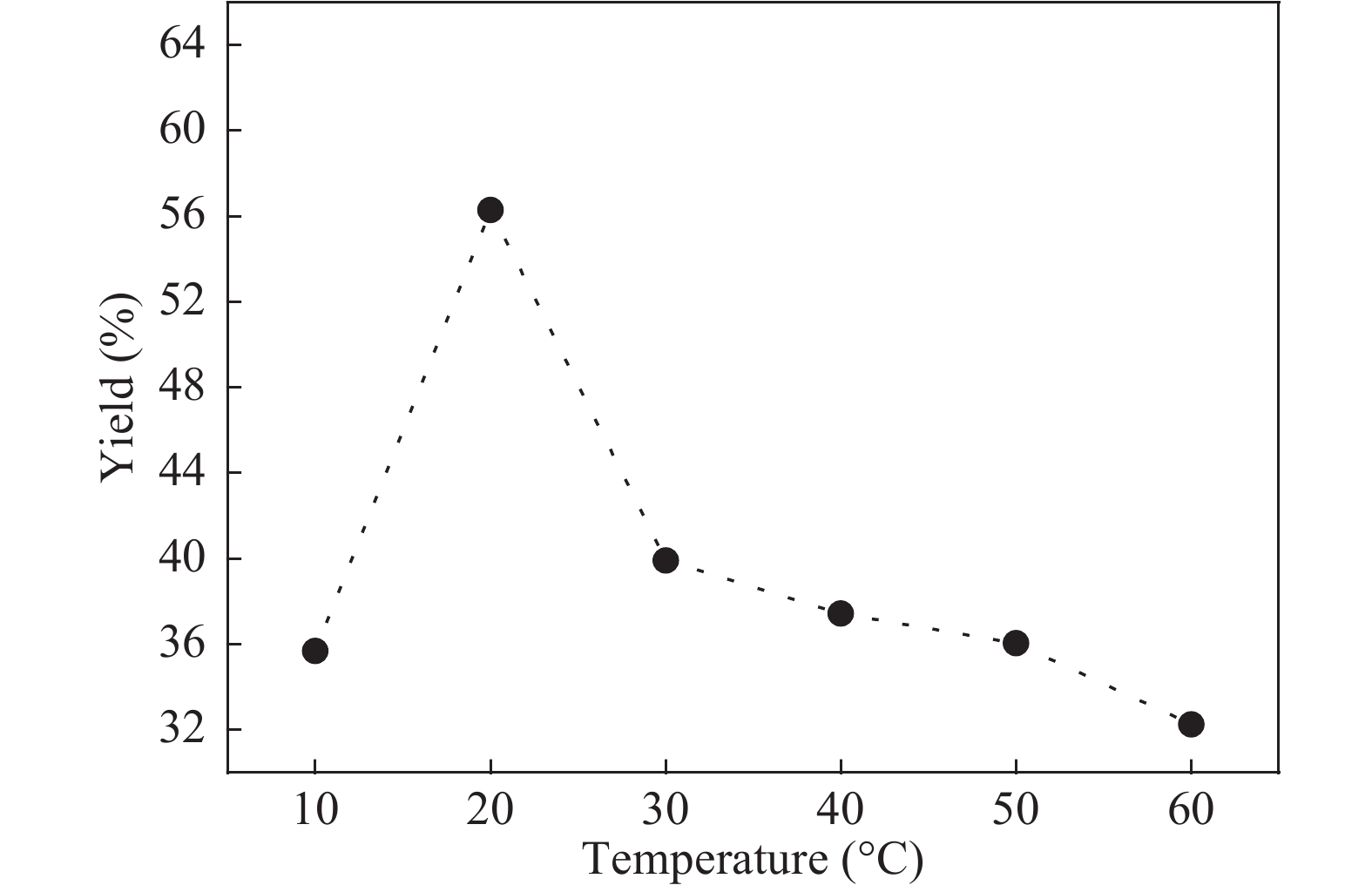
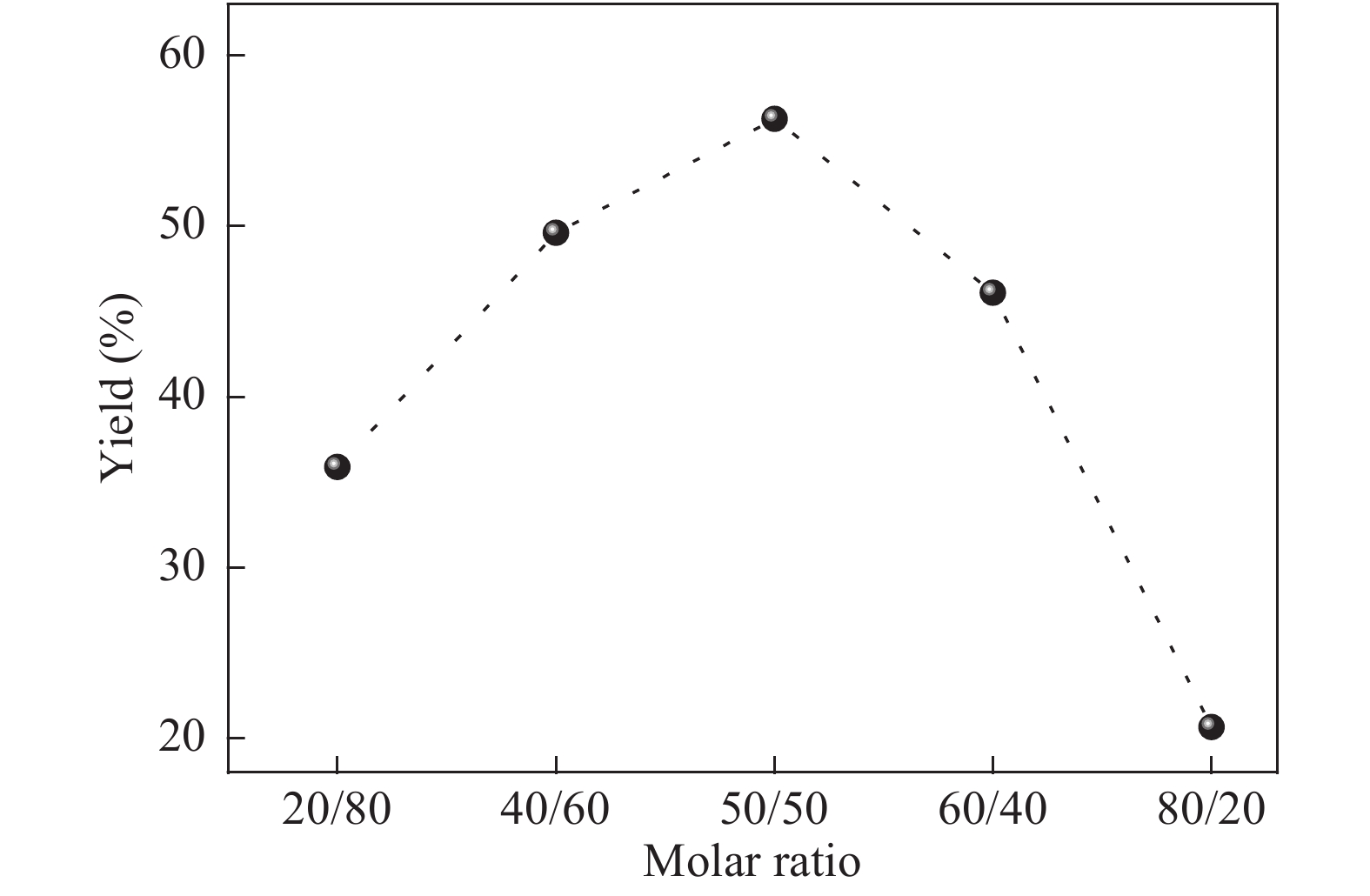
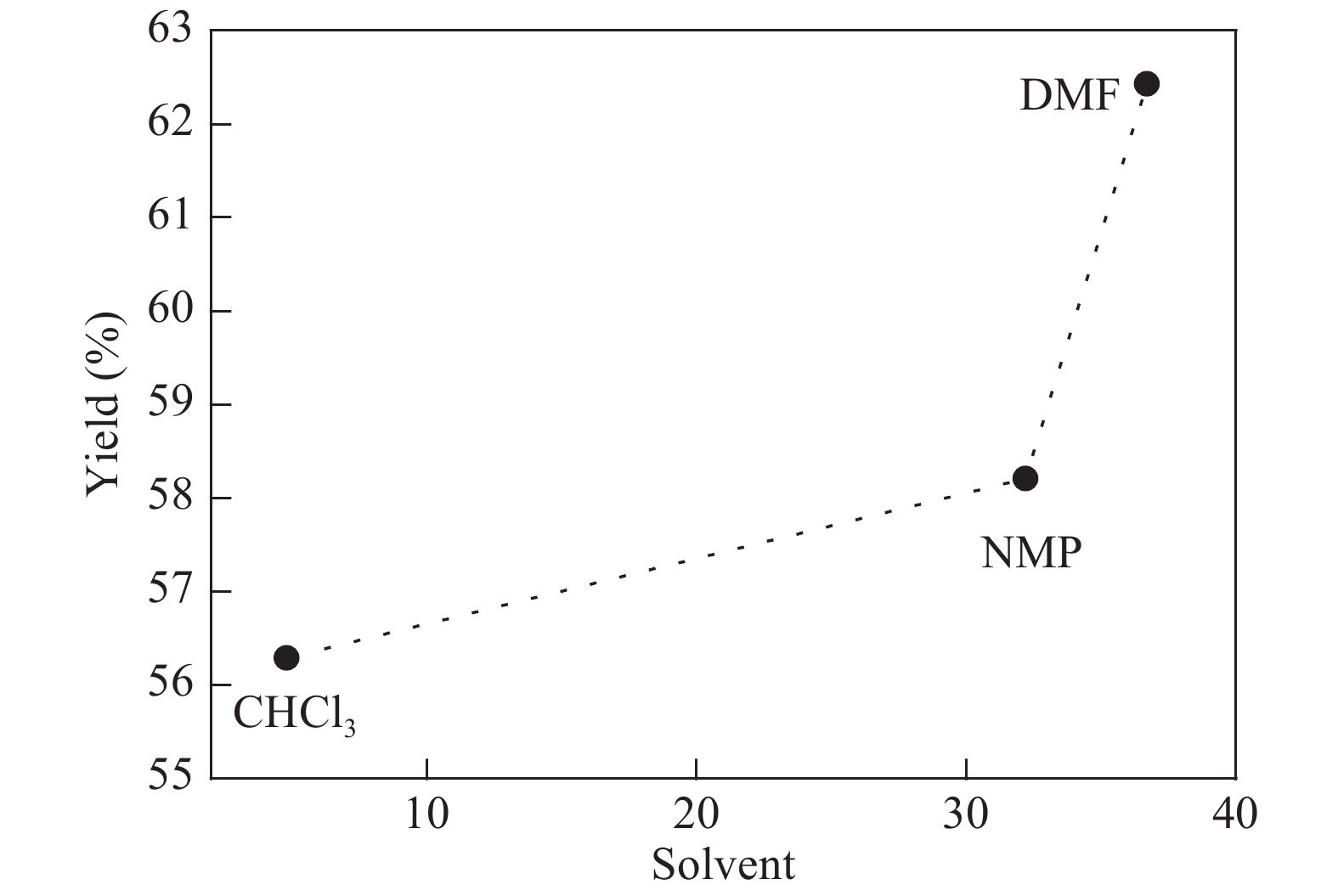
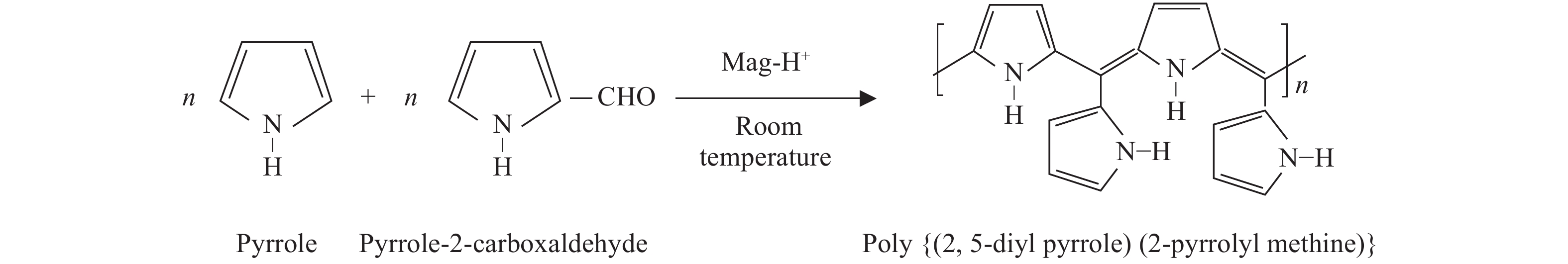
The proposed technique to synthesise poly {(2,5-diyl pyrrole) (2-pyrrolyl methine)} (PPPM) copolymer by condensation of pyrrole and pyrrole-2-carboxaldehyde monomers catalyzed by Maghnite-H+ is introduced. The protons are exchanged with Maghnite-H+, which is available in the form of a montmorillonite silicate clay sheet. The effect of several parameters such as time and temperature of copolymerization, [pyrrole]/[pyrrole-2-carboxaldehyde] molar ratio, amount of Maghnite-H+, and solvent on the produced poly (2,5-diyl pyrrole-2-pyrrolyl methine) semiconductor copolymer material (yield%) was investigated. The synthesized PPPM copolymer was characterized using nuclear magnetic resonance, Fourier transform infrared, and ultraviolet–visible spectroscopy. The results show that the synthesized copolymer using the copolymerization technique is a real organic copolymer consisting of two monomers units (i.e, pyrrole and pyrrole-2-carboxaldehyde). Also, the synthesized copolymer is more soluble than polypyrrole in most of the commonly used organic solvents. Hence, copolymerization of pyrrole with pyrrole-2-carboxaldehyde will overcome the insolubility of polypyrrole. In addition, the resultant copolymer exhibits good film formability. The produced copolymer has several potential applications in the field of rechargeable batteries, sensors, capacitors, light emitting diodes, optical displays, and solar cells. -
References
[1] Hwang L S, Ko J M, Rhee H W, et al. A polymer humidity sensor. Synth Met, 1993, 55[2] Slater J M, Watt E J, Freeman J, et al. Conducting polymers for biosensors. Application to new glucose sensors and entrapped into polypyrrole, and absorbed on poly (3-methylthiophene). Analyst, 1992, 117: 1265 doi: 10.1039/an9921701265[3] Martin R, Liang W, Menon V. Electronically conductive polymers as chemically-selective layers in membrane based separations. Synth Met, 1993, 55: 3766[4] Kudoh Y. Intrinsically conducting polymers. London: Kluwer Academic, 1993[5] Miller J S. Conducting polymers. Materials of Commerce, 1993, 5: 671[6] Sailor M J, Ginsburg E J, Gorman C B, et al. Thin-films of N-SI/poly-(CH3)3SI-cyclooctatetraene-conducting-polymer solar-cells and layered structures. Science, 1990, 249: 1146 doi: 10.1126/science.249.4973.1146[7] Gustafsson J C, Inganas O, Anderson A M. Conductive polyheterocycles as electrode materials in solid state electrochromic devices. Synth Met, 1994, 62: 17 doi: 10.1016/0379-6779(94)90193-7[8] DePaoli M A, Panero S, Passerini S, et al. Polypyrrole-dodecylsulphate: 2 × 104 cycles with an organic electrochromic material in basic medium. Adv Mater, 1990, 2: 480 doi: 10.1002/adma.19900021007[9] Kraft A, Grimsdale A C, Holmes A B. Electroluminescent conjugated polymers-seeing polymers in a new light. Angew Chem, 1998, 37: 403[10] Burroughes J H, Bradley D D C, Brown A R, et al. Light-emitting diodes based on conjugated polymers. Nature, 1990, 347: 539 doi: 10.1038/347539a0[11] Bittihm R, Ely G, Woeffier F, et al. 1987, Makromol Chem Makromol Symp, 8, 51[12] Mermillod M, Tanguy J, Petiot F. A study of chemically synthesized polypyrrole as electrode material for battery applications. J Electrochem Soc, 1986, 133: 1073 doi: 10.1149/1.2108788[13] Selampınar F, Akbulut U, Özden M Y, et al. Immobilization of invertase in conducting polymer matrices. Biomaterials, 1997, 92: 1163[14] Kızılyar N, Özden N Y, Toppare L, et al. Immobilization of invertase in conducting polypyrrole/polytetrahydrofuran graft polymer matrices. Synth Met, 1999, 104: 45 doi: 10.1016/S0379-6779(99)00033-8[15] Pellegrino J, Radebaugh R, Mattes B R. Gas sorption in polyaniline. 1. emeraldine base. Macromolecules, 1996, 29: 4985 doi: 10.1021/ma951333x[16] Sotzing G A, Reynolds J R, Steel P J. Electrochromic conducting polymers via electrochemical polymerization of bis(2-(3, 4-ethylenedioxy)thienyl) monomers. Chem Mater, 1996, 8: 882 doi: 10.1021/cm9504798[17] Lee Y, Shin D, Cho J, et al. Ionic interactions in polyacrylonitrile/polypyrrole conducting polymer composite. J Appl Polym Sci, 1998, 69: 2641 doi: 10.1002/(ISSN)1097-4628[18] Levine K L, Iroh J O. Resistance of the polypyrrole/polyimide composite by electrochemical impedance spectroscopy. J Porous Mater, 2004, 11: 87 doi: 10.1023/B:JOPO.0000027364.19392.d2[19] Yin W S, Yan T J, Gan L M, et al. Conductive composite films based on polypyrrole and crosslinked poly (styrene butyl acrylate acrylic acid). Eur J Polym, 1998, 34: 1763 doi: 10.1016/S0014-3057(98)00045-7[20] Mano V, Felisberti M I, Matencio T, et al. Thermal, mechanical and electrochemical behavior of poly (vinyl chloride)/ polypyrrole blends (PVC/PPy). Polymer, 1996, 37: 5165 doi: 10.1016/0032-3861(96)00339-4[21] Brahim S, Guiseppi-Elie A. Electroconductive hydrogels: Electrical and electrochemical properties of polypyrrole-poly(HEMA) composites. Electroanal, 2005, 17: 556 doi: 10.1002/(ISSN)1521-4109[22] Park Y H, Shin H C, Lee Y, et al. Formation of polypyrrole copolymer in PSPMS precursor film by electrochemical polymerization. Mol Cryst Liq Cryst Sci Technol A, 1999, 327: 221 doi: 10.1080/10587259908026818[23] Stanke D, Hallensleben M, Toppare L. Graftcopolymers and composites of poly(methyl methacrylate) and polypyrrole. Synthetic Metals, 1995, 73: 261 doi: 10.1016/0379-6779(95)80024-7[24] Kizilyar N, Toppare L, Onen A, et al. Conducting copolymers of polypyrrole /polytetrahydrofuran. Polym Bull, 1998, 40: 639 doi: 10.1007/s002890050302[25] Park Y H, Kim K W, Jo W H. Preparation and characterization of conducting poly(acryloyl chloride)-g-polypyrrole copolymer. Polym Adv Technol, 2002, 13: 670 doi: 10.1002/(ISSN)1099-1581[26] Tarkuc S, Sahin E, Toppare L, et al. Synthesis, characterization and electrochromic properties of a conducting copolymer of pyrrole functionalized polystyrene with pyrrole. Polymer, 2006, 47: 2001 doi: 10.1016/j.polymer.2006.01.072[27] Kizilyar N, Toppare L, Onen A, et al. Synthesis of conducting PPy/pTHF copolymers. Polym Sci, 1999, 71: 713[28] Jerome C, Martinot L, Louette P, et al. Synthesis of new (pyrrole-g-ε-caprolactone) copolymers. Macromol Symp, 2000, 153: 305 doi: 10.1002/(ISSN)1521-3900[29] Borole D D, Kapadi U R, Mahulikar P P, et al. Electrochemical synthesis and characterization of conduct-ing copolymer: poly(o-anisidine-co-o-toluidine). Mater Lett, 2006, 60: 2447 doi: 10.1016/j.matlet.2006.01.014[30] Mu S L. Poly(aniline-co-o-aminophenol) nanostructured network: Electrochemical controllable synthesis and electrocatalysis. ElectrochiActa, 2006, 51: 3434 doi: 10.1016/j.electacta.2005.09.039[31] Dhanalakshmi K, Saraswathi R. Electrochemical preparation and characterization of conducting copolymers: poly(pyrrole-co-indole). J Mater Sci, 2001, 36: 4107 doi: 10.1023/A:1017988015634[32] Belbachir M, Bensaoula A. Composition and method for catalysis using bentonites. US Patent, 2001[33] Yahiaoui A, Belbachir M. Ring-opening polymerization of styrene oxide with Maghnite-H+ as ecocatalyst. J Appl Polym Sci, 2006, 100: 1681 doi: 10.1002/(ISSN)1097-4628[34] Hachemaoui A, Yahiaoui A, Belbachir M. Synthesis and characterization of water soluble poly(N-acetyl) iminoethylene and poly(ethyleneimine) by ion-exchanged clay montmorillonite. J Appl Polym Sci, 2006, 102: 3741 doi: 10.1002/(ISSN)1097-4628[35] Meghabar R, Megherbi A, Belbachir M. Maghnite-H+, an ecocatalyst for cationic polymerization of N-vinyl-2-pyrrolidone. Polymer, 2003, 44: 4097 doi: 10.1016/S0032-3861(03)00400-2[36] Boutaleb N, Benyoucef A, Salavagione H J, et al. Electrochemical behaviour of conducting polymers obtained into clay-catalyst layers. An in situ Raman spectroscopy study. Eur J Polym, 2006, 424: 733[37] Mourad A H I, Akkad R O, Soliman A A, et al. Characterization of thermally treated and untreated polyethylene-polypropylene blends using DSC, TGA and IR techniques. Plastics, Rubber and Composites. Macromol Eng, 2009, 38: 265[38] Babaghayou M I, Mourad A H I, Lorenzo V, et al. Photodegradation characterization and heterogeneity evaluation of the exposed and unexposed faces of stabilized and unstabilized LDPE films. Mater Des, 2016, 111: 279 doi: 10.1016/j.matdes.2016.08.065[39] Babaghayou M I, Mourad A H I, Lorenzo V L, et al. Anisotropy evolution of low density polyethylene greenhouse covering films during their service life. Polym Test, 2018, 66: 146 doi: 10.1016/j.polymertesting.2018.01.007[40] Dehbi A, Mourad A H I. Durability of mono-layer versus tri-layers LDPE films used as greenhouse cover: Comparative study. Arab J Chem, 2016, 9: S282 doi: 10.1016/j.arabjc.2011.04.010[41] Djakhdane K, Dehbi A, Mourad A H I, et al. The effect of sand wind, temperature and exposure time on tri-layer polyethylene film used as greenhouse roof. Macromol Eng, 2016, 45(8): 346[42] Belfedal A, Bouizem Y, Sib J D, et al. Films thickness effect on structural and optoelectronic properties of hydrogenated amorphous germanium (a-Ge:H). J Non-Cryst Solids, 2012, 358: 1404 doi: 10.1016/j.jnoncrysol.2012.03.019[43] Larbi F, Belfedal A, Sib J D, et al. Density of states in intrinsic and n/p-doped hydrogenated amorphous and microcrystalline silicon. J Modern Phys, 2011, 2: 1030 doi: 10.4236/jmp.2011.29124[44] Tiffour I, Dehbi A, Mourad A H I, et al. Synthesis and characterization of a new organic semiconductor material. Mater Chem Phys, 2016, 178: 49 doi: 10.1016/j.matchemphys.2016.04.054[45] Rahmani A, Harrane A, Belbachir M. 1H-NMR spectra of conductive, anticorrosive and soluble polyaniline exchanged by an eco-catalyst layered (Maghnite-H+). World J Chem, 2013, 8: 20[46] Nan A, Craciunescu I, Turcu R, et al. Synthesis and characterization of new functionalised pyrrole copolymers. J Optoelectron Adv Mater, 2008, 10: 2265 -
Proportional views





 DownLoad:
DownLoad: