| Citation: |
A. Narjis, A. Outzourhit, A. Aberkouks, M. El Hasnaoui, L. Nkhaili. Structural and thermoelectric properties of copper sulphide powders[J]. Journal of Semiconductors, 2018, 39(12): 122001. doi: 10.1088/1674-4926/39/12/122001
****
A Narjis, A Outzourhit, A Aberkouks, M El Hasnaoui, L Nkhaili, Structural and thermoelectric properties of copper sulphide powders[J]. J. Semicond., 2018, 39(12): 122001. doi: 10.1088/1674-4926/39/12/122001.
|
Structural and thermoelectric properties of copper sulphide powders
DOI: 10.1088/1674-4926/39/12/122001
More Information
-
Abstract
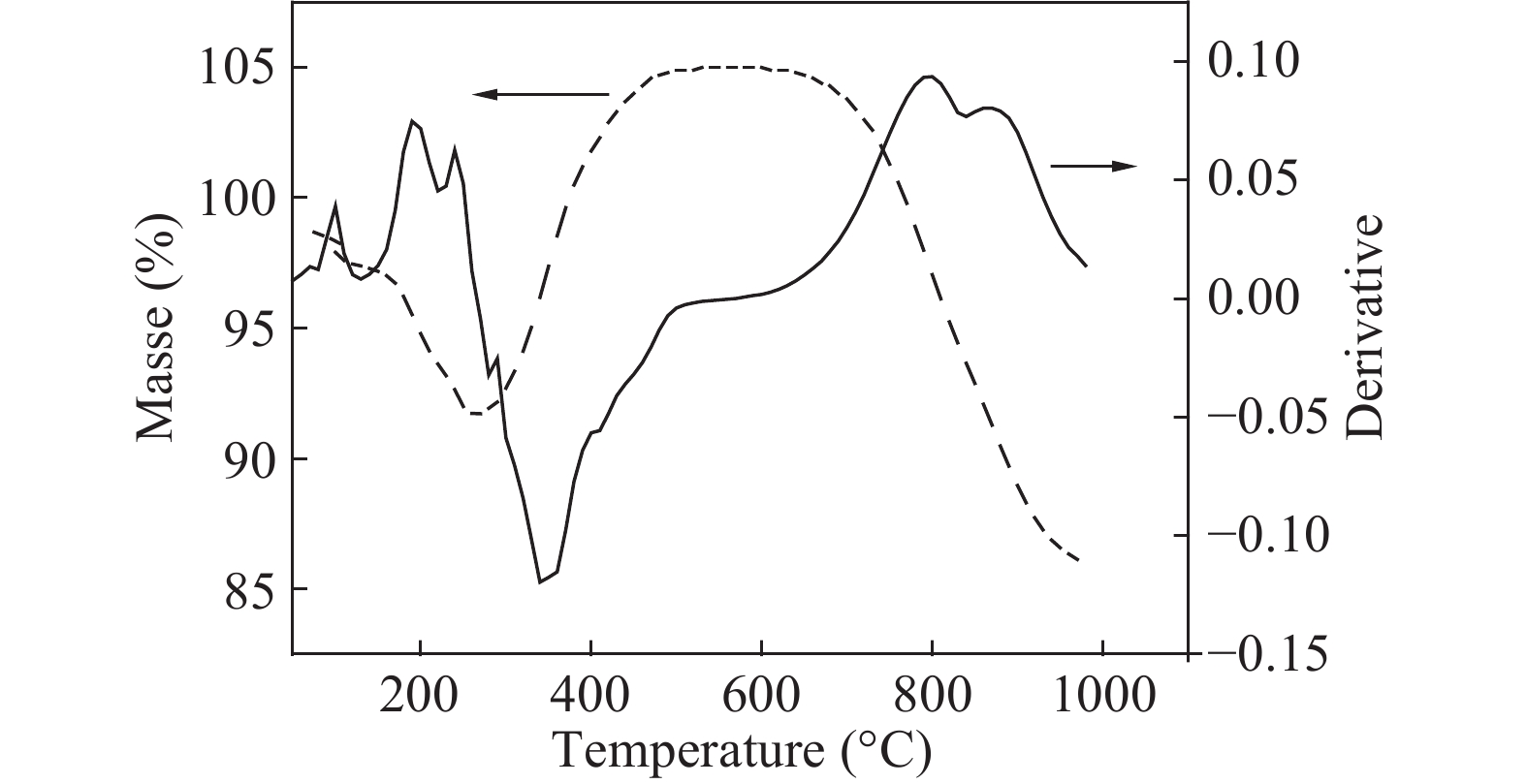
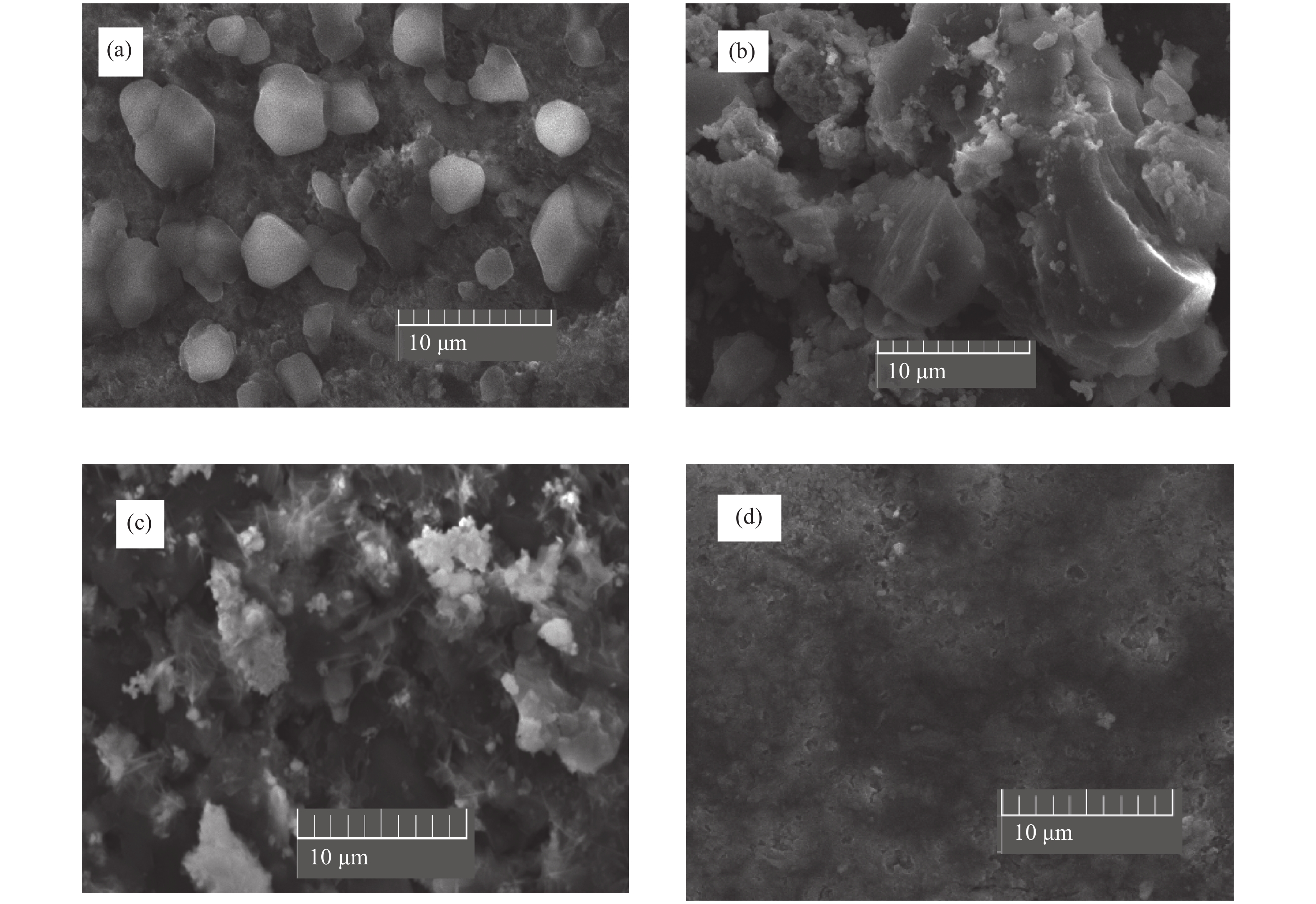
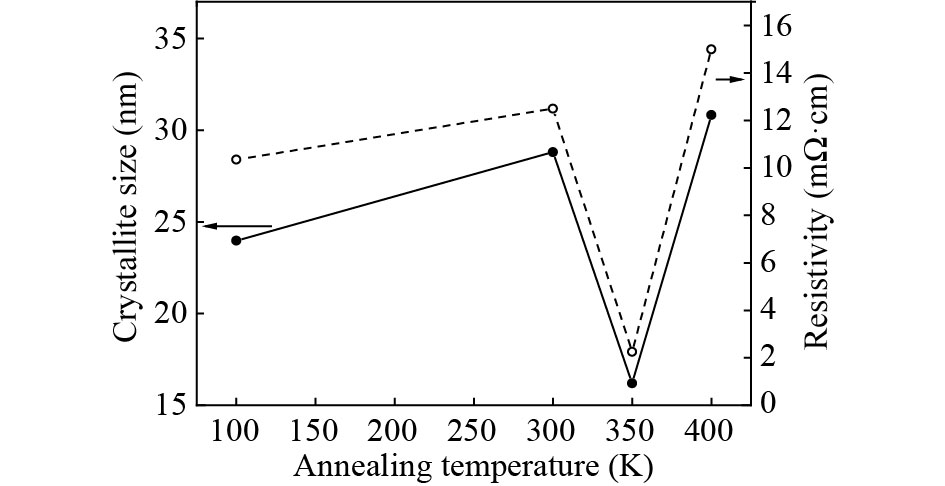
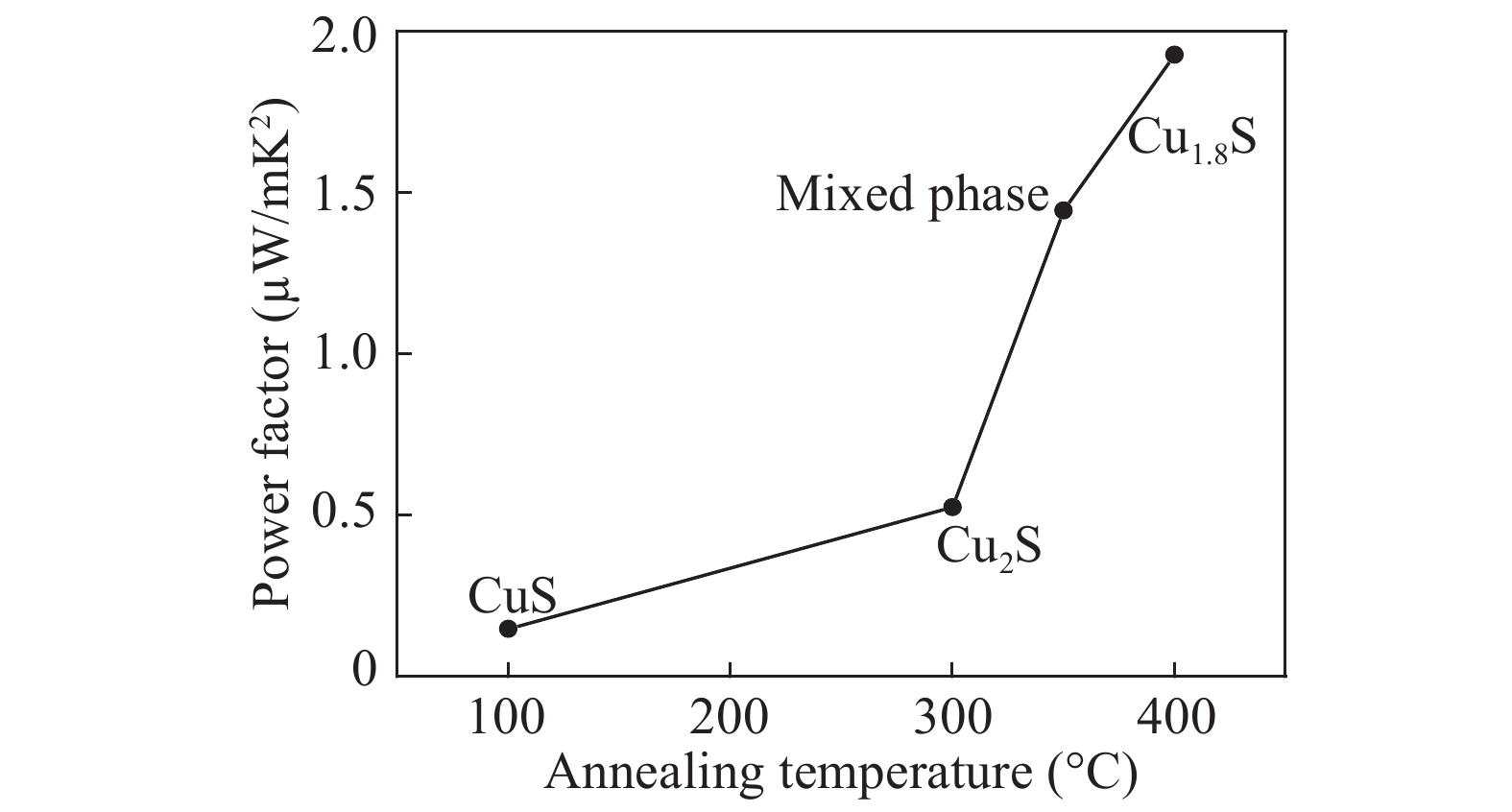
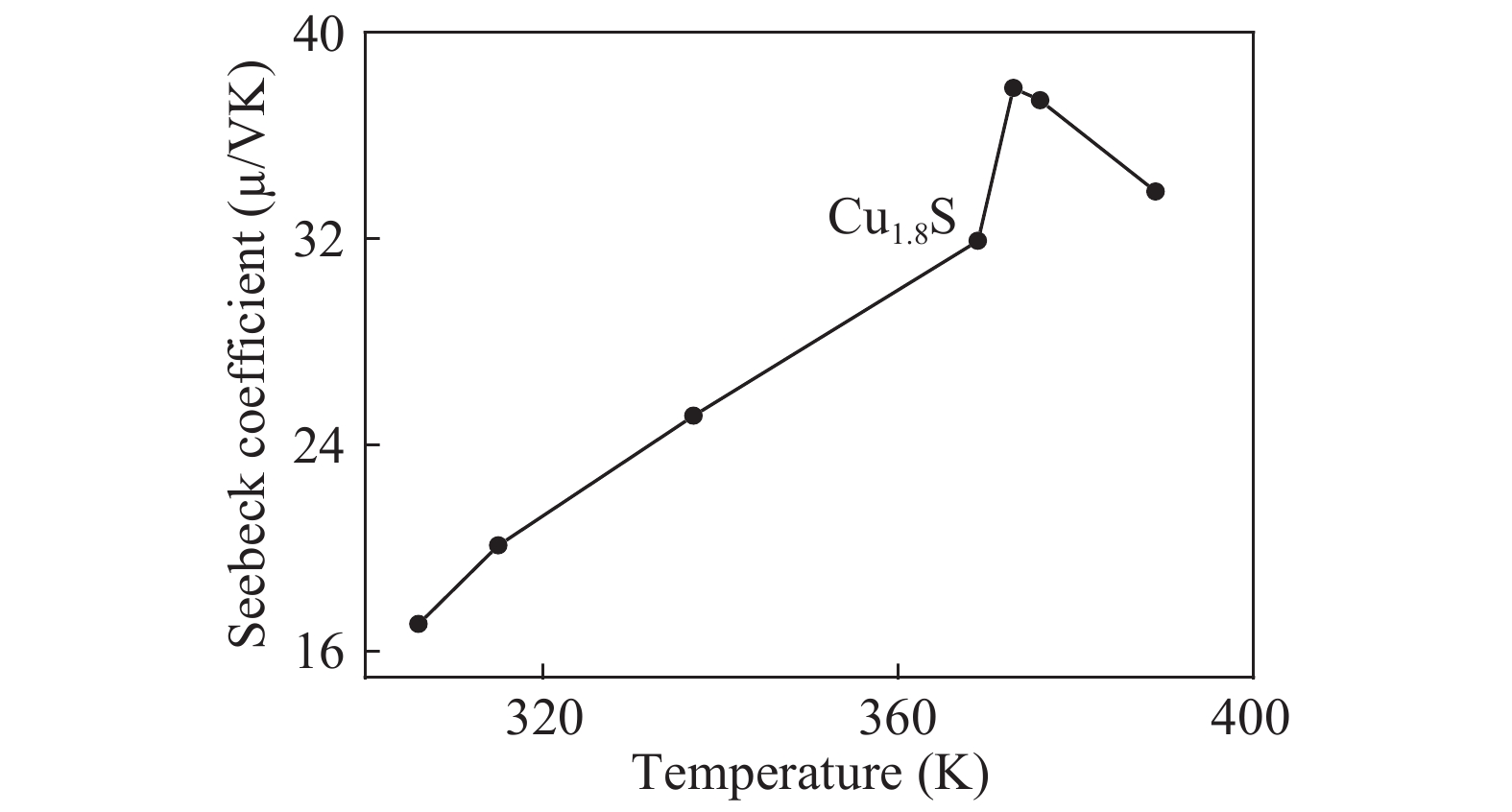
Over the past few years, Cu-based materials have been intensively studied focusing on their structural and thermoelectric properties. In this work, copper sulphide powders were synthesized by the sol-gel method. The chemical composition and the morphological properties of the obtained samples were analyzed by X-ray diffraction, differential thermal analysis, and scanning electron microscopy. It is shown that the decomposition from one phase to another can be obtained by annealing. The electrical resistivity and the crystallite size were found to be strongly affected by the phase transition. Thermoelectric analyses showed that the digenite phase exhibits the highest power factor at room temperature. The Seebeck coefficient of the compound Cu1.8S shows a pronounced peak at the γ–β transition temperature. This behavior was statistically explained in terms of a dramatic increase in the disorder in the atoms-carriers ensemble. -
References
[1] Goldsmid H J. Introduction to thermoelectricity. Berlin, Heidelberg: Springer, 2010[2] Heremans J P, Jovovic V, Toberer E S, et al. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science, 2008, 321: 554 doi: 10.1126/science.1159725[3] Pei Y Z, Shi X, LaLonde A, et al. Convergence of electronic bands for high performance bulk thermoelectrics. Nature, 2011, 473: 66 doi: 10.1038/nature09996[4] Sales B C, Mandrus D, Williams R K. Filled skutterudite antimonides: a new class of thermoelectric materials. Science, 1996, 272: 1325 doi: 10.1126/science.272.5266.1325[5] Hicks L D, Dresselhaus M S. Thermoelectric figure of merit of a one-dimensional conductor. Phys Rev B, 1993, 47(24): 1272[6] Hochbaum A I, Chen R K, Delgado R D, et al. Enhanced thermoelectric performance of rough silicon nanowires. Nature, 2008, 451: 163 doi: 10.1038/nature06381[7] Ge Z H, Zhang B P, Chen Y X, et al. Synthesis and transport property of Cu1.8S as a promising thermoelectric compound. Chem Commun, 2011, 47: 12697 doi: 10.1039/c1cc16368j[8] Mulla R, Rabinal M K. Large-scale synthesis of copper sulfide by using elemental sources via simple chemical route. Ultrasonics Sonochemistry, 2017, 39: 528 doi: 10.1016/j.ultsonch.2017.05.027[9] He Y, Day T, Zhang T, et al. High thermoelectric performance in non-toxic earth-abundant copper sulfide. Adv Mater, 2014, 26: 3974 doi: 10.1002/adma.201400515[10] Ramya M, Ganesan S. Annealing effects on resistivity properties of vacuum evaporated Cu2S thin films. IJPAP, 2010, 6: 243[11] Nair M T S, Guerrero L, Nair P K. Conversion of chemically deposited CuS thin films to Cu1.8S and Cu1.96S by annealing. Semicond Sci Technol, 1998, 13: 1164 doi: 10.1088/0268-1242/13/10/019[12] Sabah F A, Ahmed N M, Hassan Z, et al. Effect of annealing on the electrical properties of CuxS thin films. Procedia Chemistry, 2016, 19: 15 doi: 10.1016/j.proche.2016.03.005[13] Okamoto K, Kawai S. Electrical conduction and phase transition of copper sulfides. Jpn J Appl Phys, 1973, 12: 1130 doi: 10.1143/JJAP.12.1130[14] Riyaz S, Parveen A, Azam A. Microstructural and optical properties of CuS nanoparticles prepared by sol–gel route. Perspectives in Science, 2016, 8: 632 doi: 10.1016/j.pisc.2016.06.041[15] Mishra A, Bhattacharjee S, Anwar S. Simple appratus to measure Seebeck coefficient up to 900 K. Measurement, 2015, 68: 295 doi: 10.1016/j.measurement.2015.03.005[16] Beretta D, Bruno P, Lanzani G, et al. Reliable measurement of the Seebeck coefficient of organic and inorganic materials between 260 K and 460 K. Rev Sci Instrum, 2015, 86: 075104 doi: 10.1063/1.4926885[17] Srinivas B, Kumar B G, Muralidharan K. Chemical. Stabilizer free copper sulphide nanostructures for rapid photocatalytic decomposition of rhodamine B. J Mol Catal A, 2015, 410: 8 doi: 10.1016/j.molcata.2015.08.028[18] Thanikaikarasan S T. Characterization of electrodeposited copper sulphide thin films. J New Mater Electrochem Syst, 2010, 13: 29[19] Kundu J, Pradhan D. Influence of precursor concentration, surfactant and temperature on the hydrothermal synthesis of CuS: structural, thermal and optical properties. New J Chem, 2013, 37: 1470 doi: 10.1039/c3nj41142g[20] Narjis A, Outzourhit A, Aberkouks A, et al. Spectroscopic study and thermoelectric properties of a mixed phase copper sulfide lamellas. J Alloys Compd, 2018, 762: 46 doi: 10.1016/j.jallcom.2018.05.183[21] Nafees M, Ali S, Idrees S, et al. A simple microwave assists aqueous route to synthesis CuS nanoparticles and further aggregation to spherical shape. Appl Nanosci, 2013, 3: 119 doi: 10.1007/s13204-012-0113-9[22] Nafees M, Ikram M, Ali S. Thermal behavior and decomposition of copper sulfide nanomaterial synthesized by aqueous sol method. Dig J Nanomater Biostruct, 2015, 10: 635[23] Will G, Hinze E, Rahman A, et al. Crystal structure analysis and refinement of digenite, Cu1.8S, in the temperature range 20 to 500 °C under controlled sulfur partial pressure. Eur J Mineral, 2002, 14: 591 doi: 10.1127/0935-1221/2002/0014-0591[24] Li J F, Liu W S, Zhao L D, et al. High-performance nanostructured thermoelectric materials. NPG Asia Mater, 2010, 2: 152 doi: 10.1038/asiamat.2010.138[25] Pop A E, Batin M N, Popescu V. The influence of annealing temperature on copper sulphide CuxS obtained by chemical precipitation. Powder Metallurgy Progress, 2011, 11(3/4): 197[26] Quintana-Ramirez P V, Arenas-Arrocena M C, Santos-Cruz J, et al. Growth evolution and phase transition from chalcocite to digenite in nanocrystalline copper sulfide: Morphological, optical and electrical properties. Beilstein J Nanotechnol, 2014, 5: 1542 doi: 10.3762/bjnano.5.166[27] Avinash B S, Chaturmukha V S, Jayanna H S, et al. Effect of particle size on band gap and DC electrical conductivity in TiO2 nanomaterial. AIP Conf Proc, 2016, 1728: 020426 doi: 10.1063/1.4946477[28] Koshy J, George K C. Annealing effects on crystallite size and band gap of CuO nanparticles. Int J Nanosci Nanotechnol, 2015, 6(1): 1[29] Mulder B J. Optical properties of crystals of cuprous sulfides (chalcosite, djurleite, Cu1.9S, and digenite). Phys Status Solidi A, 1972, 13: 79 doi: 10.1002/(ISSN)1521-396X[30] Brown D R, Kasper T D, Borup A, et al. Phase transition enhanced thermoelectric figure-of-merit in copper chalcogenides. APL Mater, 2013, 1: 052107 doi: 10.1063/1.4827595[31] Kubo R, Yokota M, Nakajima S. Statistical-mechanical theory of irreversible processes. II. response to thermal disturbance. J Phys Soc Jpn, 1957, 12: 1203 doi: 10.1143/JPSJ.12.1203[32] Chaikin P M, Beni G. Thermopower in the correlated hopping regime. Phys Rev B, 1976, 13: 647 doi: 10.1103/PhysRevB.13.647[33] Rockwood A L. Relationship of thermoelectricity to electronic entropy. Phys Rev A, 1984, 30: 2843 doi: 10.1103/PhysRevA.30.2843 -
Proportional views






 DownLoad:
DownLoad: