| Citation: |
Lizina Khatua, Rajashree Sahoo, Pravakar Satapathy, Rudrashish Panda, Susanta Kumar Das. Visible light photocatalytic dye decomposition behaviour of solid state reaction grown Zn2TiO4 nanoparticles[J]. Journal of Semiconductors, 2018, 39(12): 123002. doi: 10.1088/1674-4926/39/12/123002
****
L Khatua, R Sahoo, P Satapathy, R Panda, S K Das, Visible light photocatalytic dye decomposition behaviour of solid state reaction grown Zn2TiO4 nanoparticles[J]. J. Semicond., 2018, 39(12): 123002. doi: 10.1088/1674-4926/39/12/123002.
|
Visible light photocatalytic dye decomposition behaviour of solid state reaction grown Zn2TiO4 nanoparticles
DOI: 10.1088/1674-4926/39/12/123002
More Information
-
Abstract
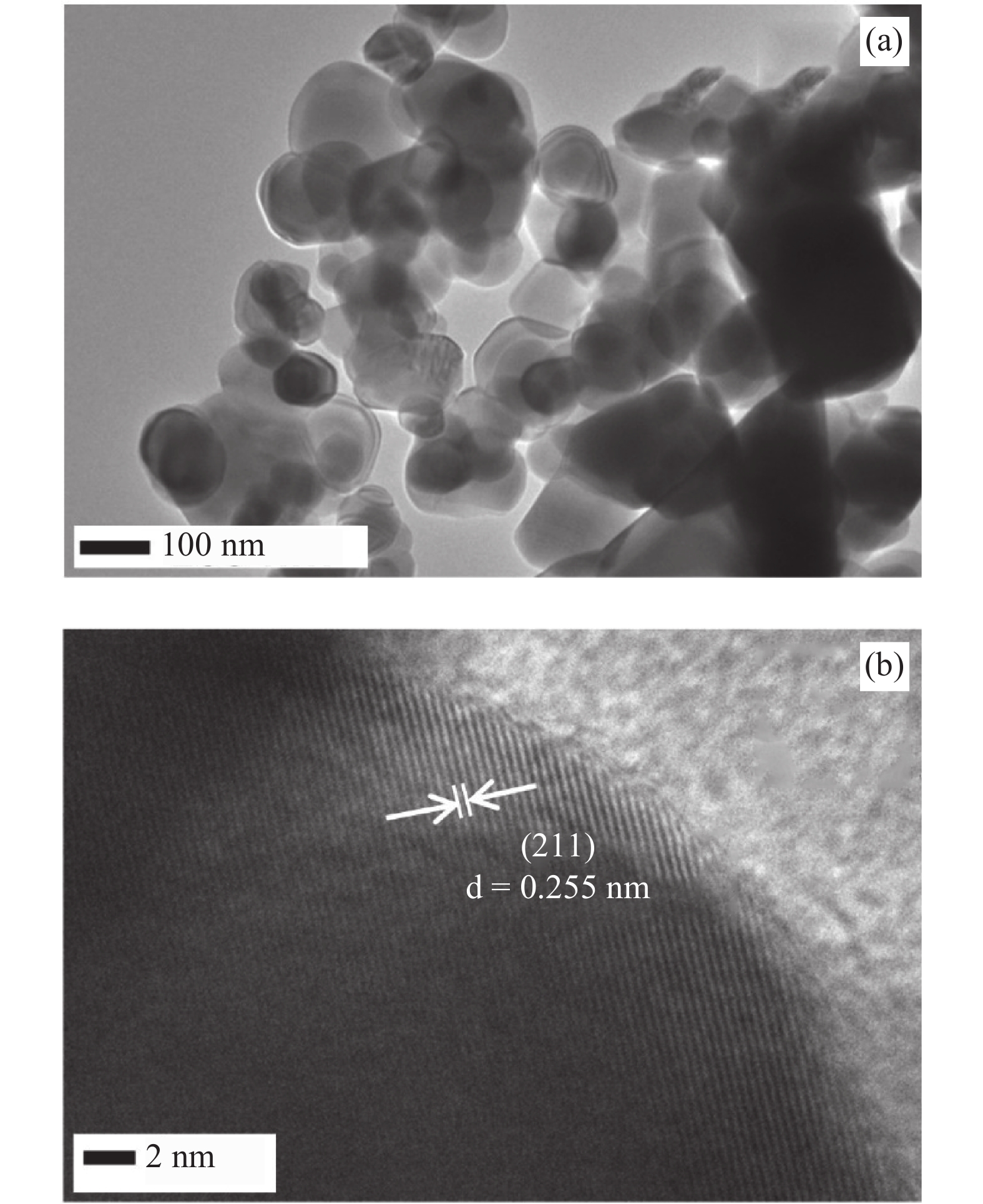
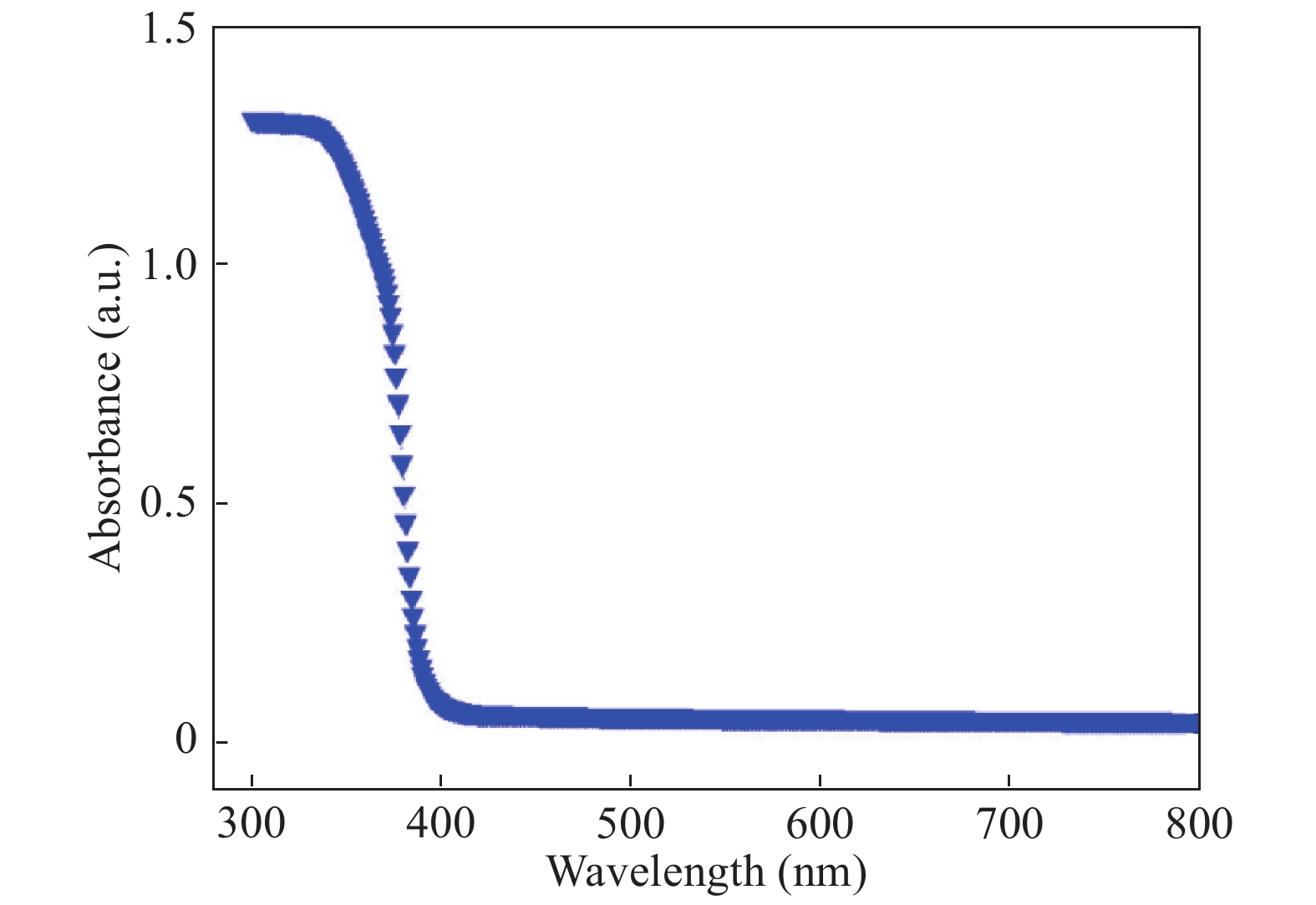
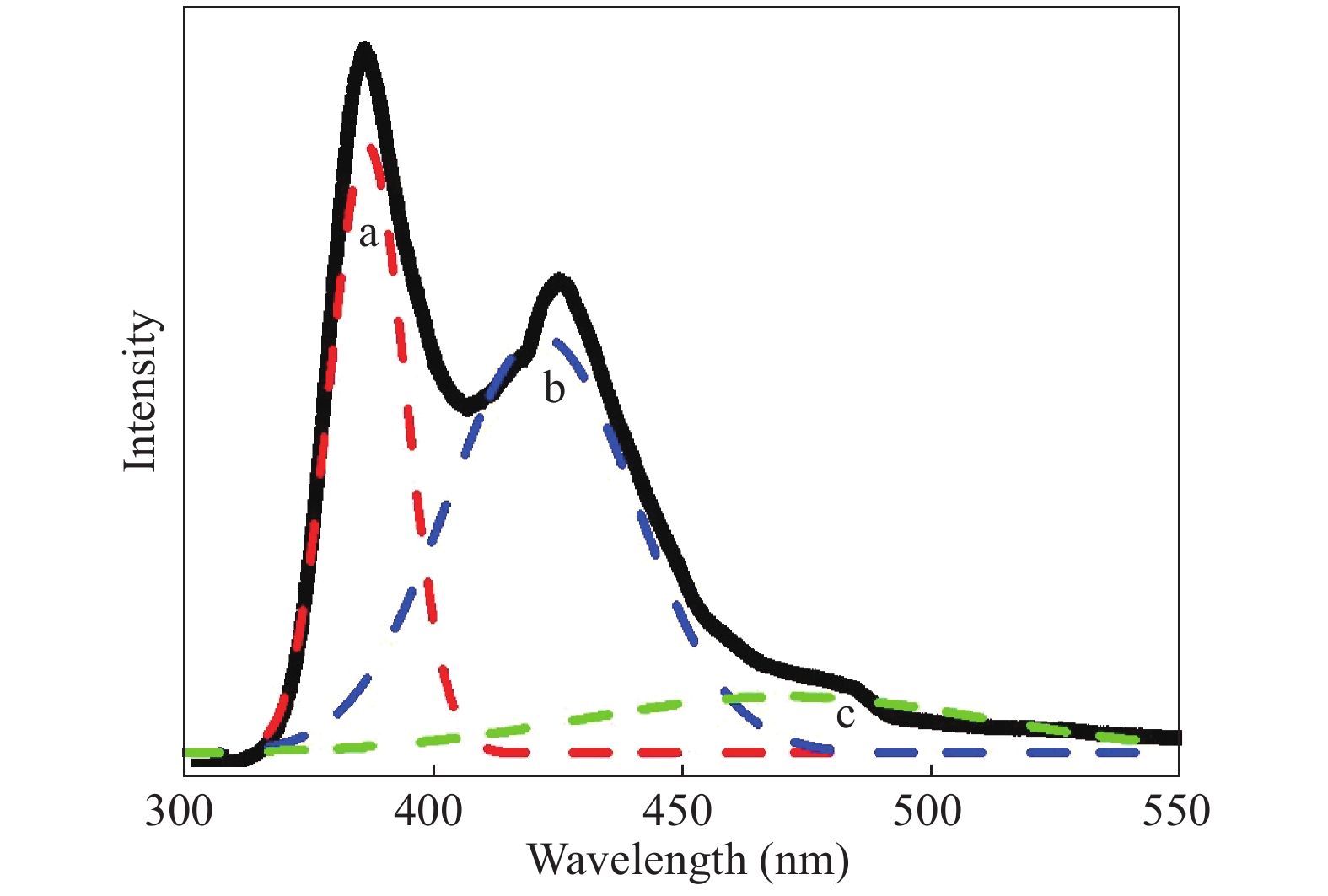
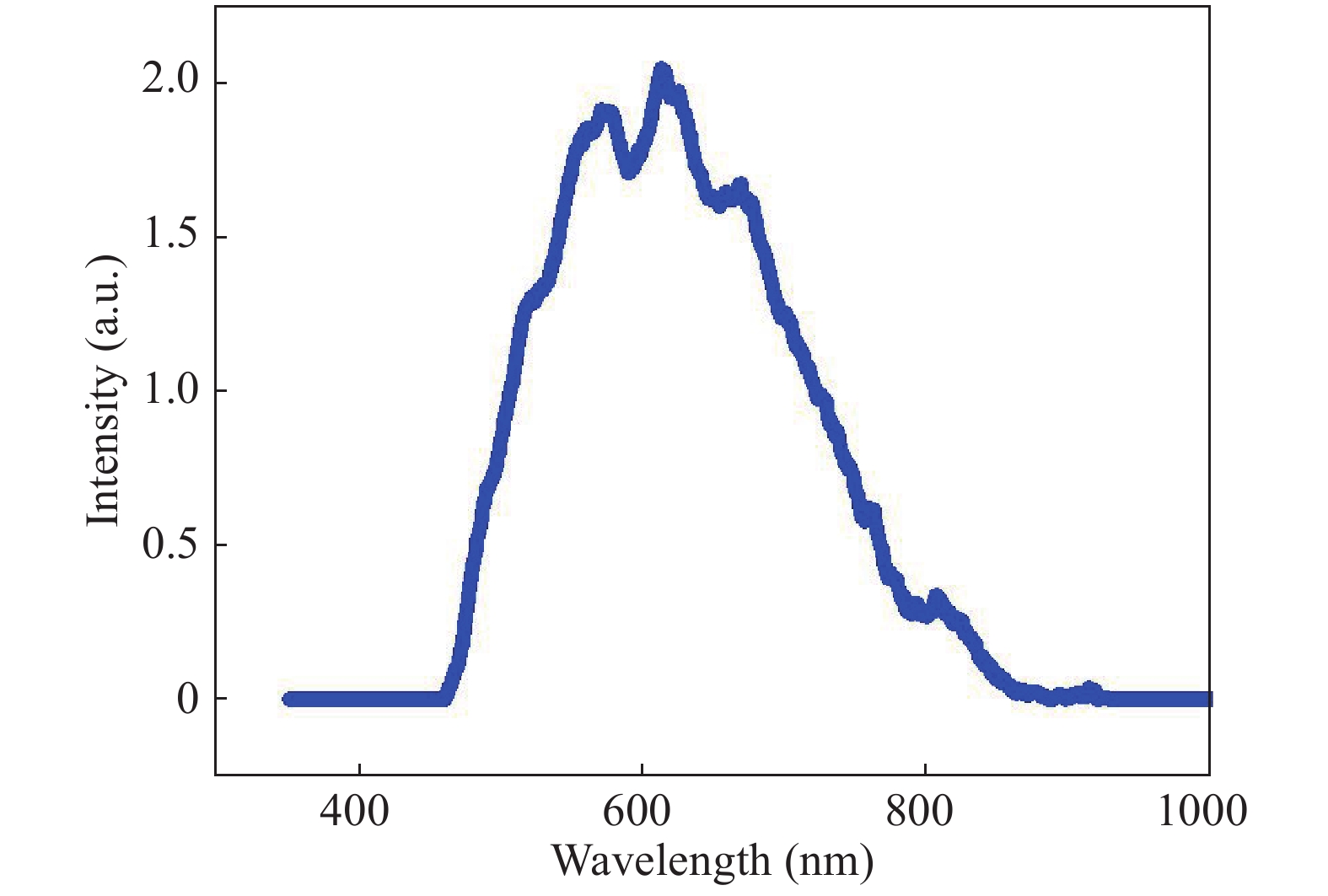
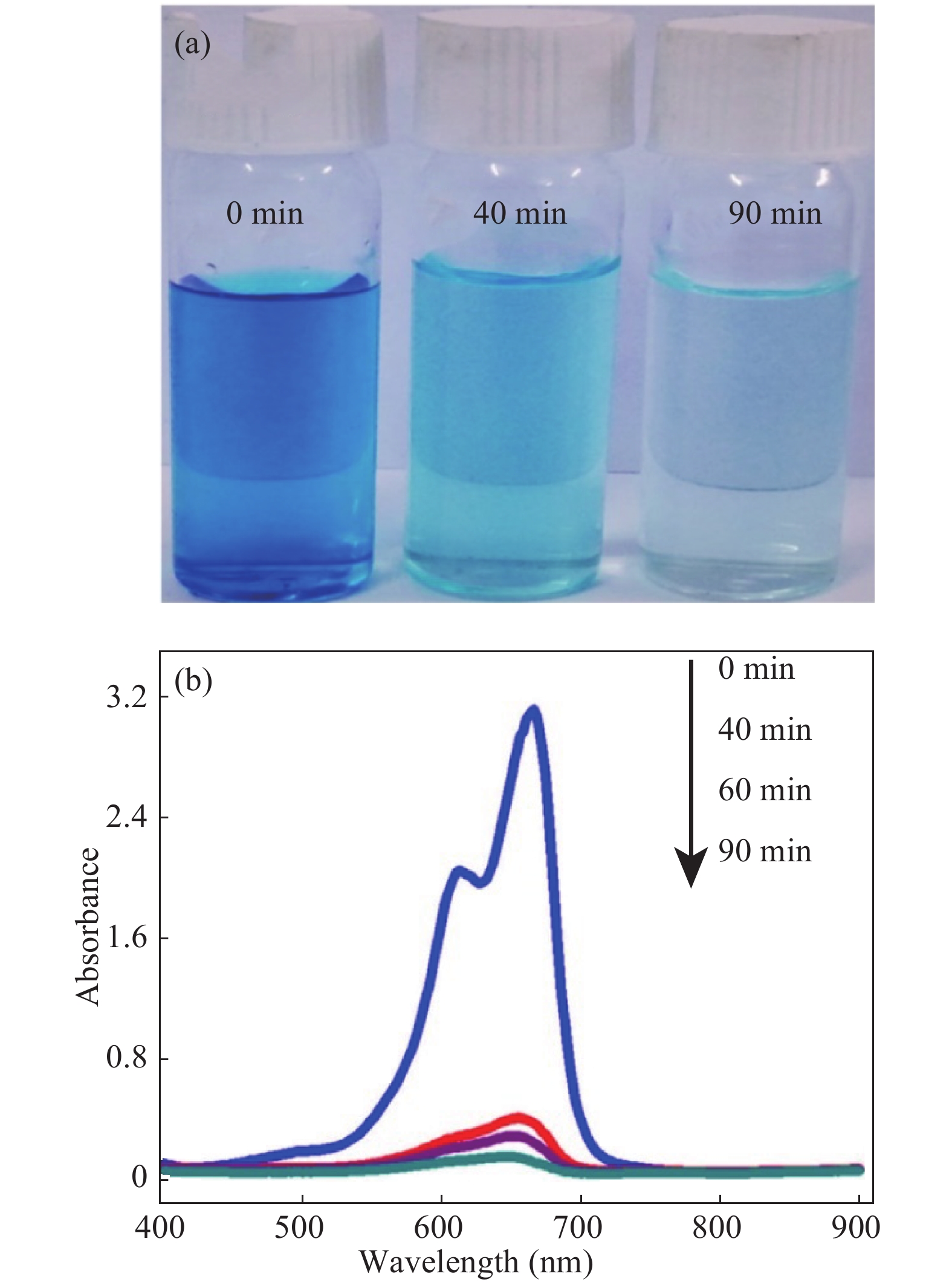
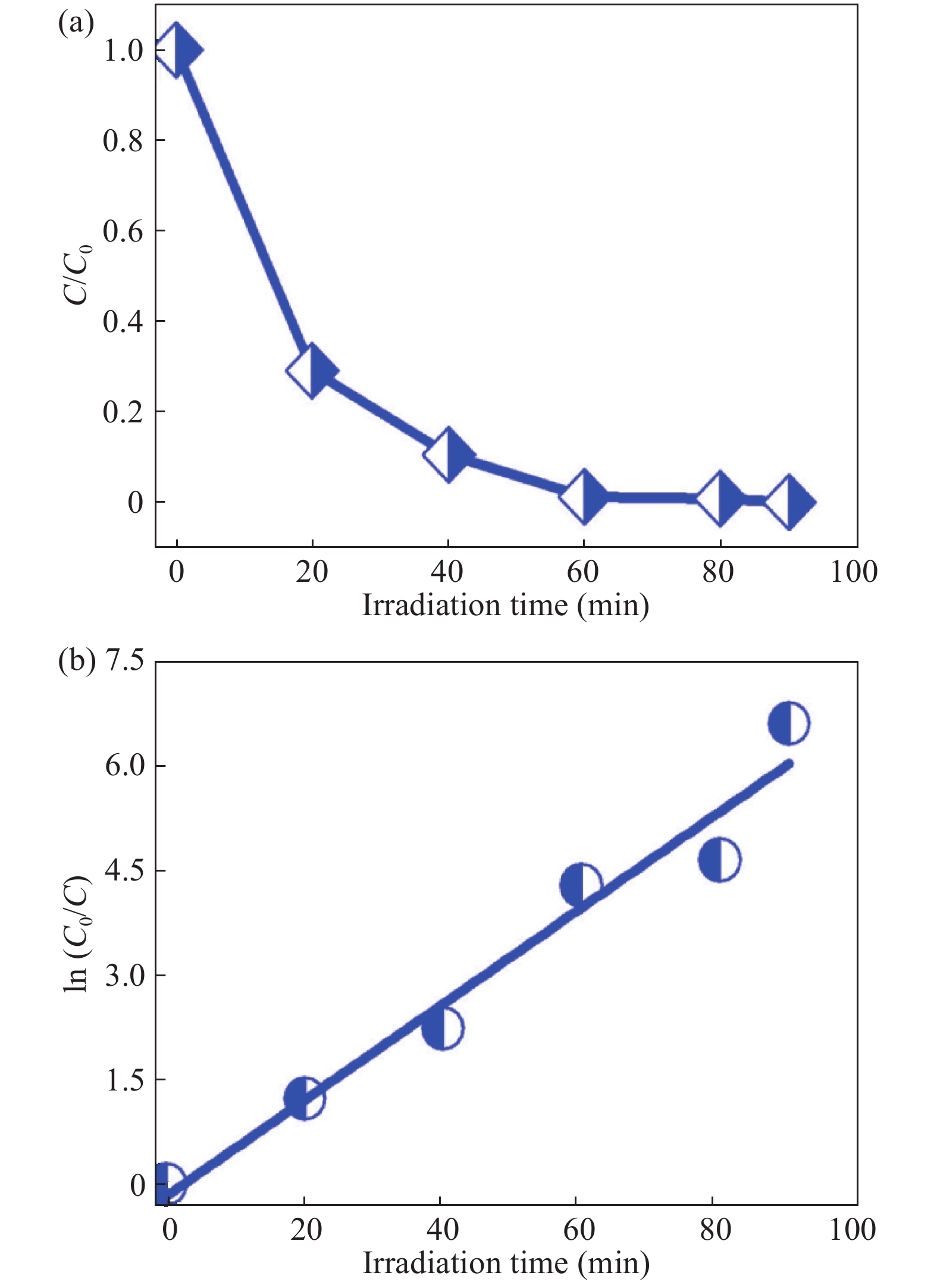
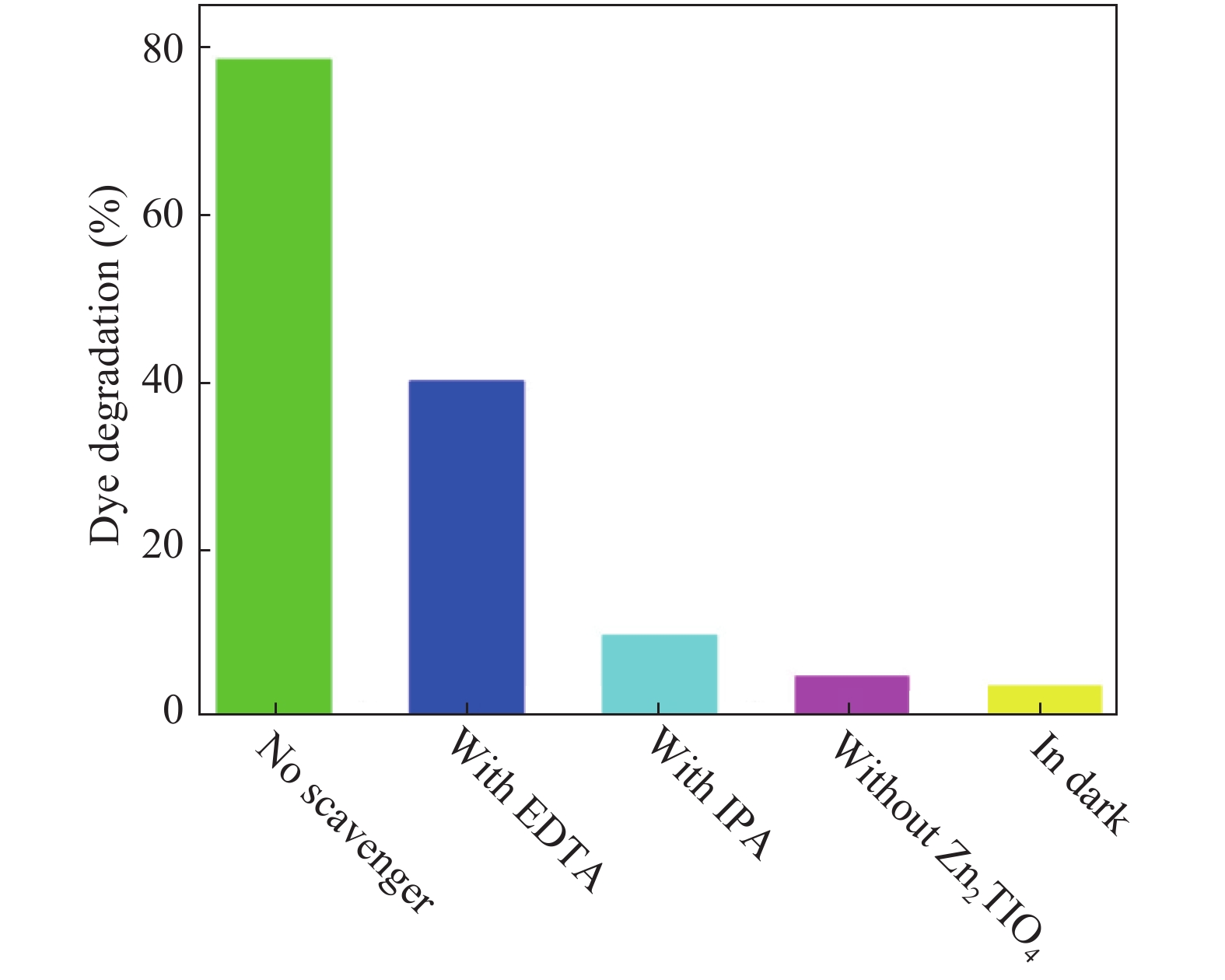
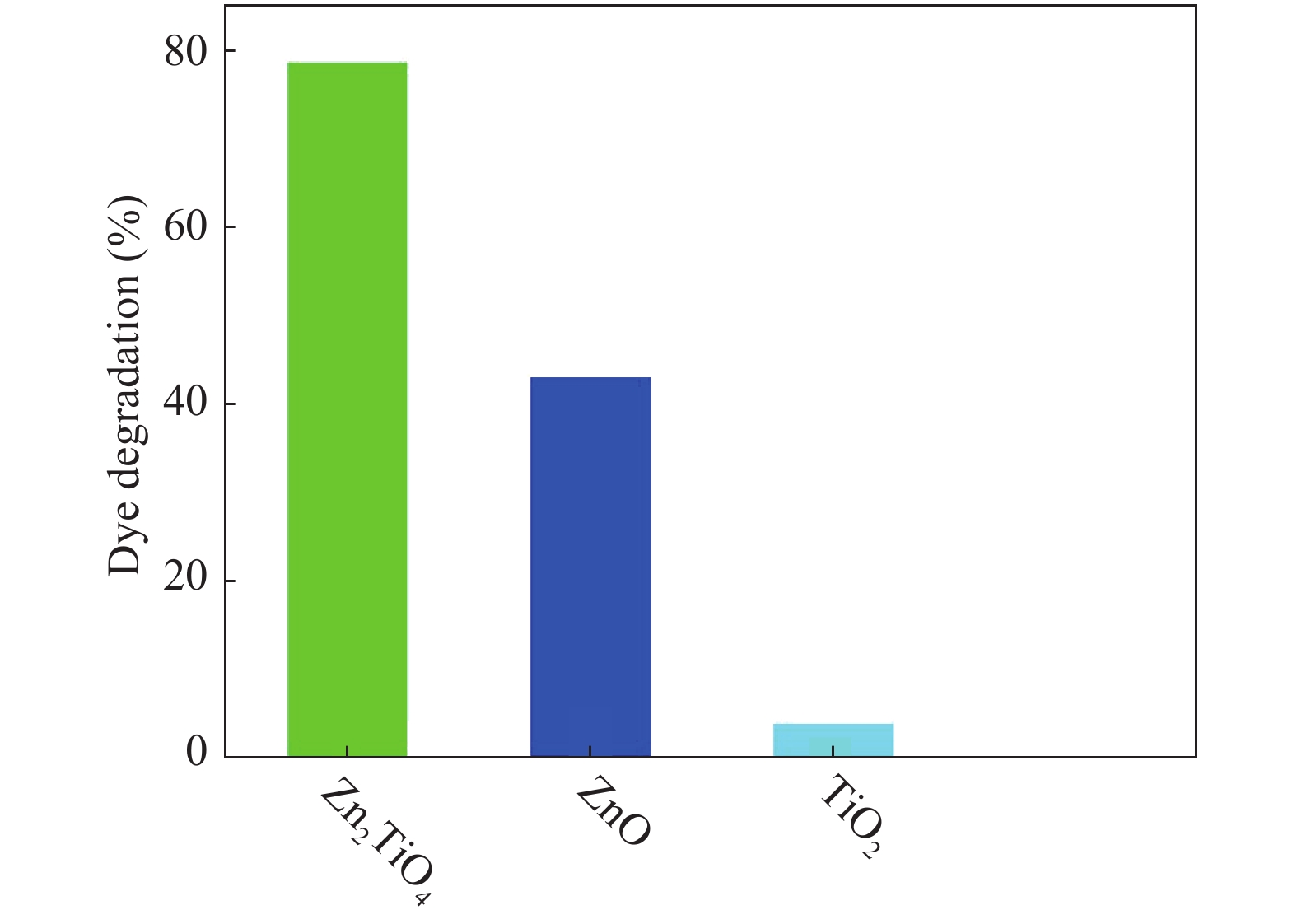
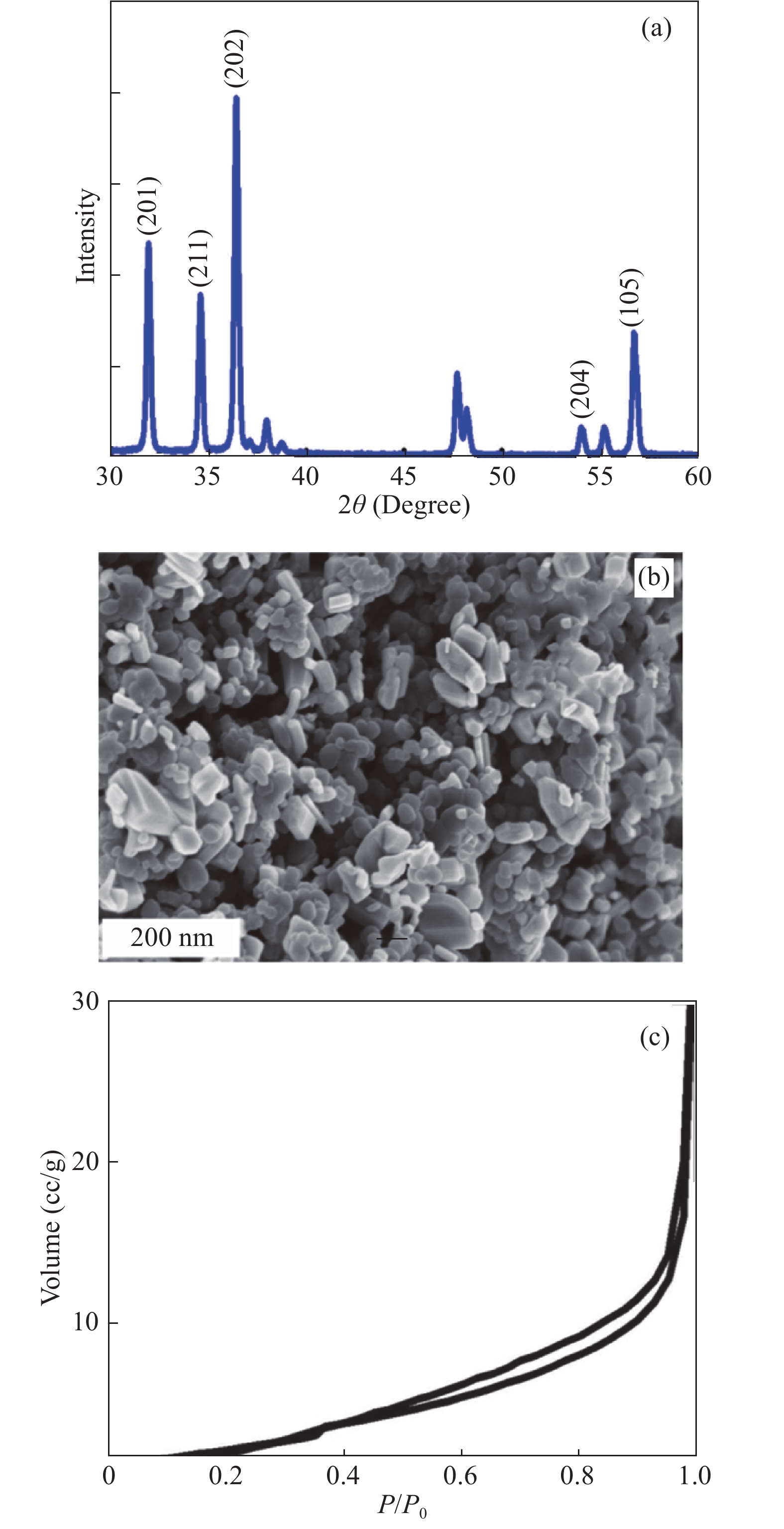
In this investigation, visible photocatalytic dye decomposition is carried out with compound semiconductor nanoparticles of zinc orthotitanate (Zn2TiO4). These nanoparticles were grown by the solid state reaction method and characterized by field emission scanning electron microscopy, transmission electron microscopy, X-ray diffraction, diffuse reflectance spectroscopy, photoluminescence study, and Brunauer–Emmett–Teller (BET) study. The BET surface area of the Zn2TiO4 nanoparticles was found to be 8.78 m2/g. The photocatalytic activity is carried out by using a 500 W halogen light source having a spectrum in the range of 450 to 860 nm and the reaction kinetics was found to be the pseudo first order. The reaction rate constant was found to be 0.069 min−1. Discussion is given on the possible mechanism of the observed visible photocatalytic dye decomposition activity. The cost of the material used is very low, so it could be very useful for visible photocatalytic dye decomposition. -
References
[1] Pare B, Singh P, Jonnalgadda S B. Artificial light assisted photocatalytic degradation of lissamine fast yellow dye in ZnO suspension in a slurry batch reactor Indian. J Chem A, 2009, 48: 1364[2] Janus M, Tryba B, Kusiak E, et al. TiO2 nanoparticles with high photocatalytic activity under visible light. Catal Lett, 2009, 128: 36 doi: 10.1007/s10562-008-9721-0[3] Lou F, Qian X, Jin Y, et al. Characterisation of water-soluble TiO2 and its photocatalytic activity under visible light. Mater Res Innov, 2015, 19: S8-693 doi: 10.1179/1432891715Z.0000000001779[4] Amini M, Ashrafi M. Photocatalytic degradation of some organic dyes under solar light irradiation using TiO2 and ZnO nanoparticles. Nanochem Res, 2016, 1: 79[5] Han J, Liu Y, Singhal N, et al. Comparative photocatalytic degradation of estrone in water by ZnO and TiO2 under artificial UVA and solar irradiation. Chem Eng J, 2012, 213: 150 doi: 10.1016/j.cej.2012.09.066[6] Cheng C, Amini A, Zhu C, et al. Enhanced photocatalytic performance of TiO2–ZnO hybrid nanostructures. Sci Rep, 2011, 5: 4181[7] Sun X, Wang S, Shen C, et al. Efficient photocatalytic hydrogen production over Rh-doped inverse spinel Zn2TiO4. Chem Cat Chem, 2016, 8: 2289[8] Borse P H, Cho C R, Lim K T, et al. Processing research comparision of Zn2TiO4 and rutile TiO2 photocatalysts for H2 production under UV and near-visible light irradiation. J Ceram Process Res, 2012, 13: 42[9] Habib M A, Shahadat M T, Bahadur N M, et al. Synthesis and characterization of ZnO–TiO2 nanocomposites and their application as photocatalysts. Int Nano Lett, 2013, 3: 5 doi: 10.1186/2228-5326-3-5[10] Jang J S, Borse P H, Lee J S, et al. Energy band structure and photocatalytic property of Fe-doped Zn2TiO4 material. Bull Kor Chem Soc, 2009, 30: 3021 doi: 10.5012/bkcs.2009.30.12.3021[11] Channei D, Inceesungvorn B, Wetchakun N, et al. Photocatalytic degradation of methyl orange by CeO2 and Fe-doped CeO2 films under visible light irradiation. Sci Rep, 2015, 4: 5757 doi: 10.1038/srep05757[12] Nikam L, Panmand R, Kadam S, et al. Enhanced hydrogen production under a visible light source and dye degradation under natural sunlight using nanostructured doped zinc orthotitanates. New J Chem, 2015, 39: 3821 doi: 10.1039/C4NJ01995D[13] Lopera A A, Velásquez A M, Chavarriaga E A, et al. Synthesis by combustion in solution of Zn2TiO4+Ag for photocatalytic and photodynamic applications in the visible. J Phys Conf Ser, 2017, 935: 12013 doi: 10.1088/1742-6596/935/1/012013[14] Zhang P, Shao C, Zhang M, et al. Controllable synthesis of Zn2TiO4@carbon core/shell nanofibers with high photocatalytic performance. J Hazard Mater, 2012, 229/230: 265 doi: 10.1016/j.jhazmat.2012.05.102[15] Iordanova R, Dimitriev Y. Mechanochemical synthesis and photocatalytic properties of zinc titanates. Bulg Chem Commun, 2011, 43: 378[16] Kaleji B, Mousaei M, Halakouie H, et al. Their photo-catalyst investigation in methylene blue degradation. Nanostructures, 2015, 5: 219[17] Arin J, Thongtem S, Phuruangrat A, et al. Template synthesis of Zn2TiO4 and Zn2Ti3O8 nanorods by hydrothermal-calcination combined processes. Mater Lett, 2017, 193: 270 doi: 10.1016/j.matlet.2017.01.142[18] Miao X, Yue X, Ji Z, et al. Nitrogen-doped carbon dots decorated on g-C3N4/Ag3PO4 photocatalyst with improved visible light photocatalytic activity and mechanism insight. Appl Catal B, 2018, 227: 459 doi: 10.1016/j.apcatb.2018.01.057[19] Chen G, Sun M, Wei Q, et al. Ag3PO4/graphene-oxide composite with remarkably enhanced visible-light-driven photocatalytic activity toward dyes in water. J. Hazard Mater, 2013, 244/245: 86 doi: 10.1016/j.jhazmat.2012.11.032[20] Khatua L, Nayak A K, Panda R, et al. Growth of significantly low dimensional zinc orthotitanate (Zn2TiO4) nanoparticles by solid state reaction method. Sci Sinter, 2018, 50: 133 doi: 10.2298/SOS1801133K[21] Guo H L, Zhu Q, Wu X L, et al. Oxygen deficient ZnO1−x nanosheets with high visible light photocatalytic activity. Nanoscale, 2015, 7: 7216 doi: 10.1039/C5NR00271K[22] Qin X, Cui L, Shao G. Preparation of ZnO–Zn2TiO4 sol composite films and its photocatalytic activities. J Nanomater, 2013, 2013: 1[23] Chen G, Ji S, Sang Y, et al. Synthesis of scaly Sn3O4/TiO2 nanobelt heterostructures for enhanced UV–visible light photocatalytic activity. Nanoscale, 2015, 7: 3117 doi: 10.1039/C4NR05749J[24] Li X, Zhang P, Jin L, et al. Efficient photocatalytic decomposition of perfluorooctanoic acid by indium oxide and its mechanism. Environ Sci Technol, 2012, 46: 5528 doi: 10.1021/es204279u[25] Liu T, Wang L, Lu X, et al. Comparative study of the photocatalytic performance for the degradation of different dyes by ZnIn2S4: adsorption, active species, and pathways. RSC Adv, 2017, 7: 12292 doi: 10.1039/C7RA00199A[26] Janitabar-Darzi S, Mahjoub A , Ghaemi A. Sol-gel periparation of ZnO/Zn2TiO4 nanocomposite for photocatalytic degradation of crystal violet. World Acad Sci Eng Technol, 2011, 52: 524[27] Wen M Q, Xiong T, Zang Z G, et al. Synthesis of MoS2/g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity for the removal of nitric oxide (NO). Opt Express, 2016, 24: 10205 doi: 10.1364/OE.24.010205[28] Huang H, Zhang J, Jiang L, et al. Preparation of cubic Cu2O nanoparticles wrapped by reduced graphene oxide for the efficient removal of rhodamine B. J Alloys Compd, 2017, 718: 112 doi: 10.1016/j.jallcom.2017.05.132[29] Ye Y, Zang Z, Zhou T, et al. Theoretical and experimental investigation of highly photocatalytic performance of CuInZnS nanoporous structure for removing the NO gas. J Catal, 2018, 357: 100 doi: 10.1016/j.jcat.2017.11.002 -
Proportional views





 DownLoad:
DownLoad: