| Citation: |
Zhong Ma, Desheng Kong, Lijia Pan, Zhenan Bao. Skin-inspired electronics: emerging semiconductor devices and systems[J]. Journal of Semiconductors, 2020, 41(4): 041601. doi: 10.1088/1674-4926/41/4/041601
****
Z Ma, D S Kong, L J Pan, Z N Bao, Skin-inspired electronics: emerging semiconductor devices and systems[J]. J. Semicond., 2020, 41(4): 041601. doi: 10.1088/1674-4926/41/4/041601.
|
Skin-inspired electronics: emerging semiconductor devices and systems
DOI: 10.1088/1674-4926/41/4/041601
More Information
-
Abstract
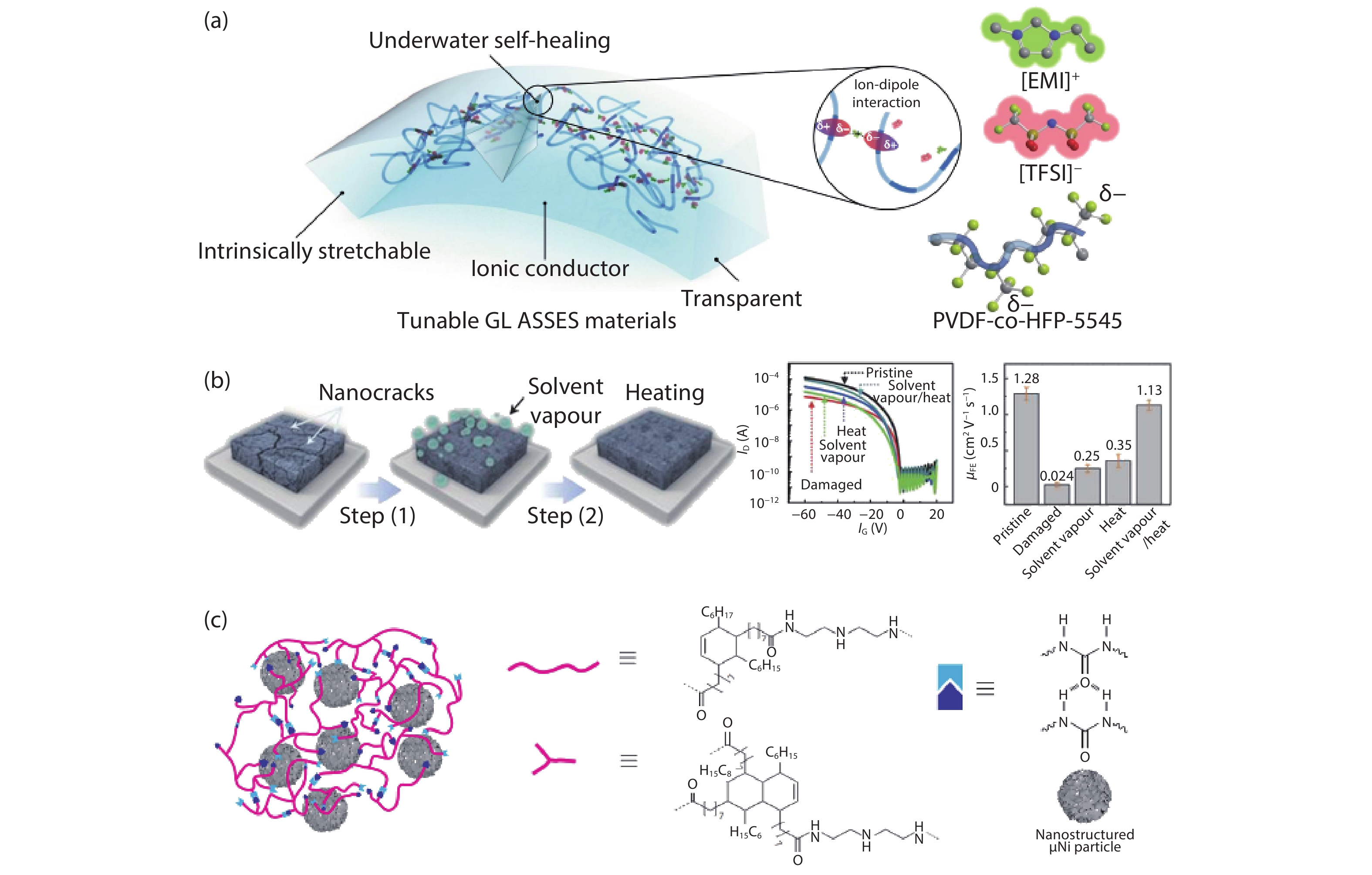
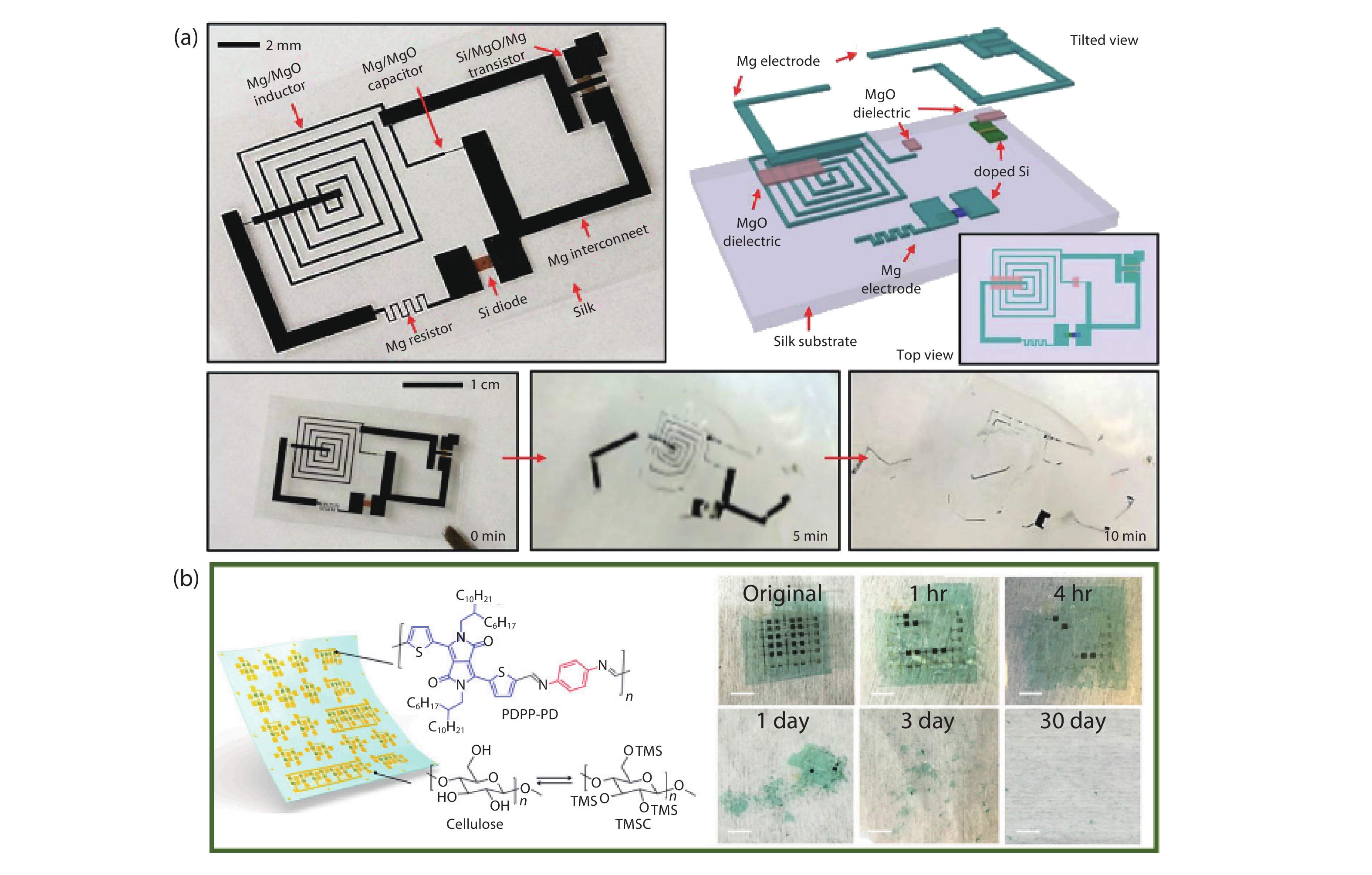
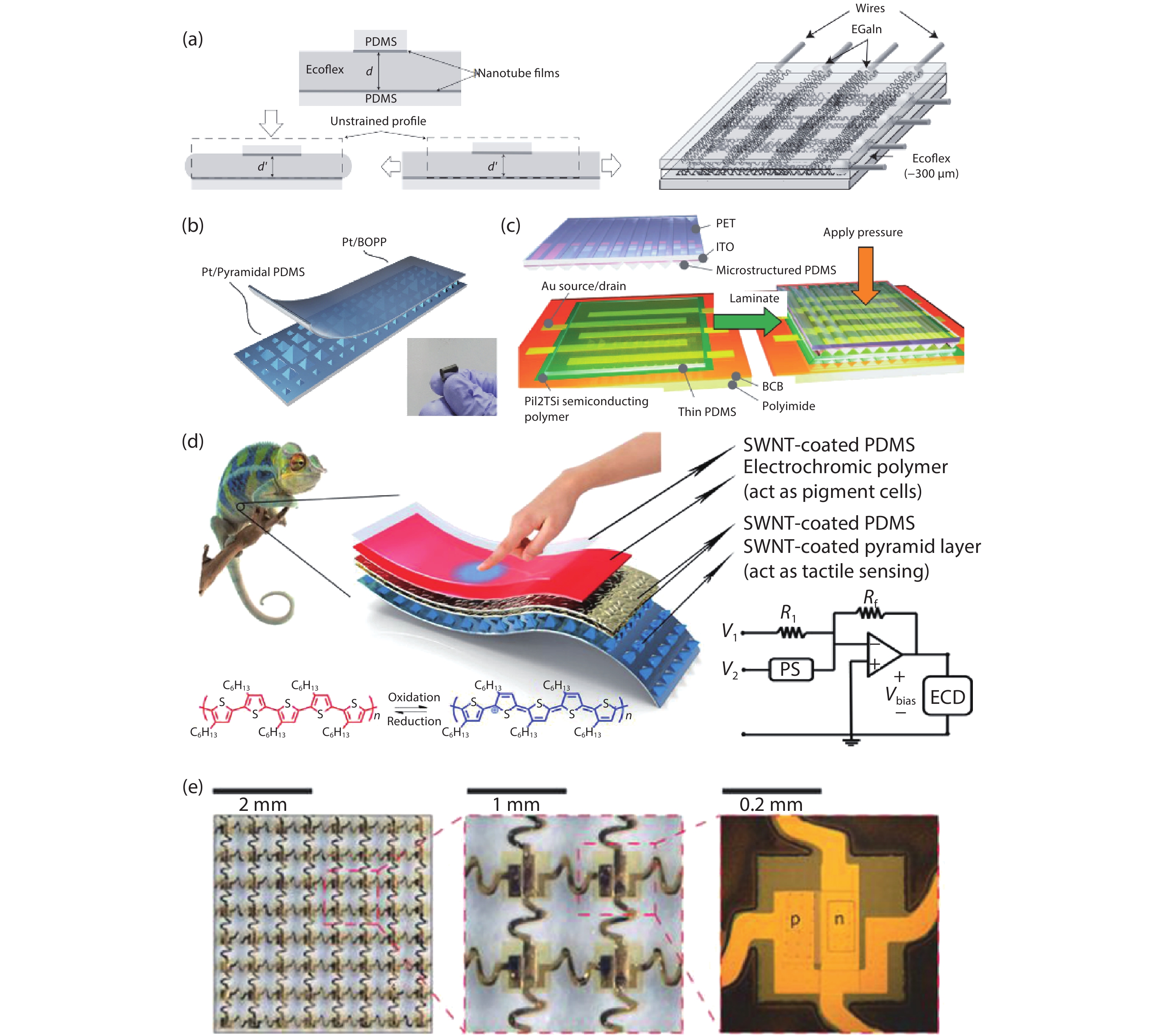
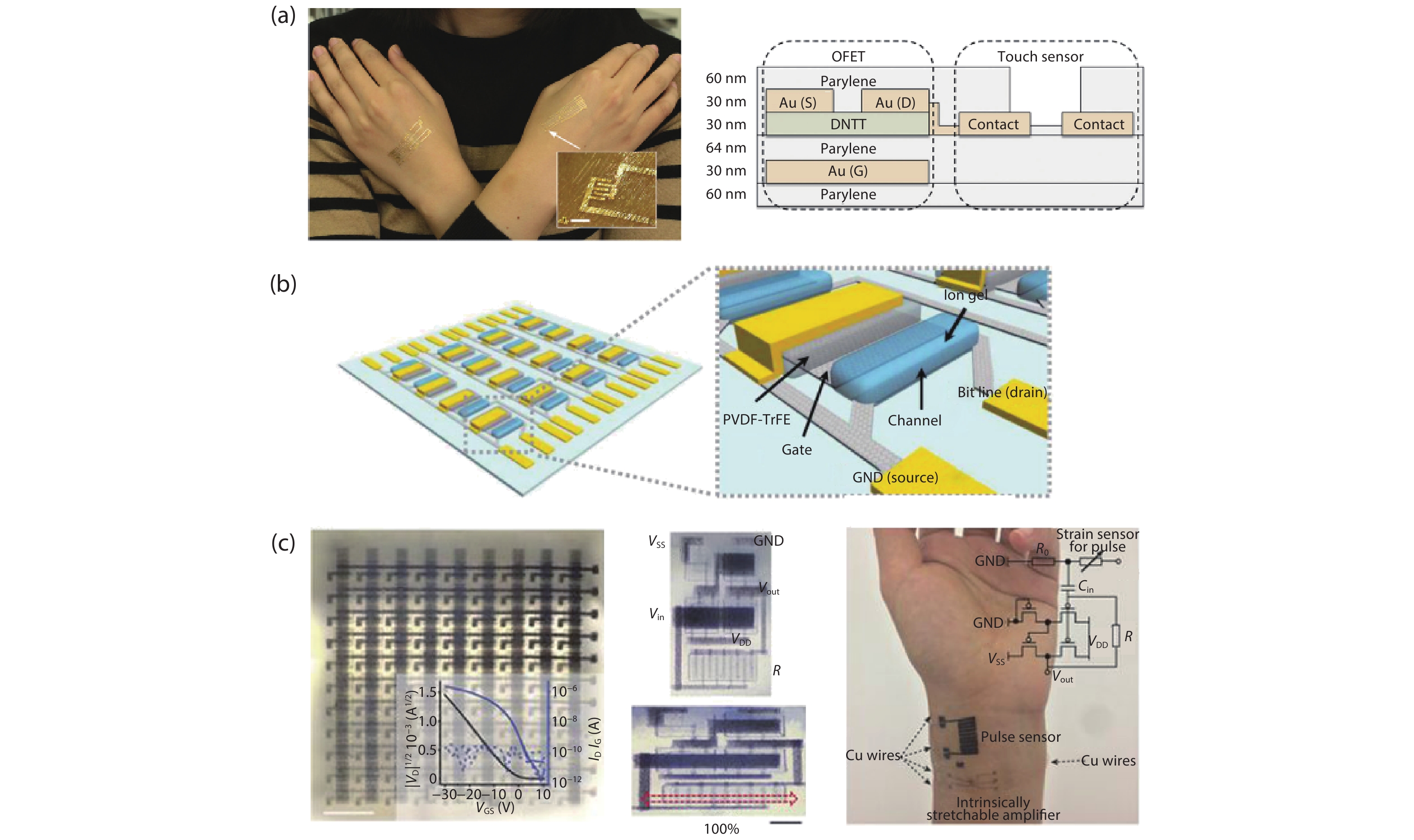
Current electronics are driven by advanced microfabrication for fast and efficient information processing. In spite of high performance, these wafer-based devices are rigid, non-degradable, and unable to autonomous repair. Skin-inspired electronics have emerged as a new class of devices and systems for next-generation flexible and wearable electronics. The technology gains inspiration from the structures, properties, and sensing mechanisms of the skin, which may find a broad range of applications in cutting-edge fields such as healthcare monitoring, human-machine interface, and soft robotics/prostheses. Practical demands have fueled the development of electronic materials with skin-like properties in terms of stretchability, self-healing capability, and biodegradability. These materials provide the basis for functional sensors with innovative and biomimetic designs. Further system-level integrations and optimizations enable new forms of electronics for real-world applications. This review summarizes recent advancements in this active area and speculates on future directions. -
References
[1] Wang S, Oh J Y, Xu J, et al. Skin-inspired electronics: an emerging paradigm. Acc Chem Res, 2018, 51(5), 1033 doi: 10.1021/acs.accounts.8b00015[2] Yang J C, Mun J, Kwon S Y, et al. Electronic skin: recent progress and future prospects for skin-attachable devices for health monitoring, robotics, and prosthetics. Adv Mater, 2019, 1904765 doi: 10.1002/adma.201904765[3] Hammock M L, Chortos A, Tee B C K, et al. The evolution of electronic skin (E-Skin): A brief history, design considerations, and recent progress. Adv Mater, 2013, 25(42), 5997 doi: 10.1002/adma.201302240[4] Ma Z, Li S, Wang H, et al. Advanced electronic skin devices for healthcare applications. J Mater Chem B, 2019, 7(2), 173 doi: 10.1039/C8TB02862A[5] Wang L, Chen D, Jiang K, et al. New insights and perspectives into biological materials for flexible electronics. Chem Soc Rev, 2017, 46(22), 6764 doi: 10.1039/C7CS00278E[6] Li T, Li Y, Zhang T. Materials, structures, and functions for flexible and stretchable biomimetic sensors. Acc Chem Res, 2019, 52(2), 288 doi: 10.1021/acs.accounts.8b00497[7] Liu Y, He K, Chen G, et al. Nature-inspired structural materials for flexible electronic devices. Chem Rev, 2017, 117(20), 12893 doi: 10.1021/acs.chemrev.7b00291[8] Wang K, Lou Z, Wang L, et al. Bioinspired interlocked structure-induced high deformability for two-dimensional titanium carbide (MXene)/natural microcapsule-based flexible pressure sensors. ACS Nano, 2019, 13(8), 9139 doi: 10.1021/acsnano.9b03454[9] Chortos A, Liu J, Bao Z. Pursuing prosthetic electronic skin. Nat Mater, 2016, 15(9), 937 doi: 10.1038/nmat4671[10] Li J, Ma Z, Wang H, et al. Skin-inspired electronics and its applications in advanced intelligent systems. Adv Intell Syst, 2019, 0(0), 1900063 doi: 10.1002/aisy.201900063[11] Lou Z, Wang L, Shen G. Recent advances in smart wearable sensing systems. Adv Mater Technol, 2018, 3(12), 1800444 doi: 10.1002/admt.201800444[12] Yao S, Swetha P, Zhu Y. Nanomaterial-enabled wearable sensors for healthcare. Adv Healthc Mater, 2018, 7(1), 1700889 doi: 10.1002/adhm.201700889[13] Edwards C, Marks R. Evaluation of biomechanical properties of human skin. Clin Dermatol, 1995, 13(4), 375 doi: 10.1016/0738-081X(95)00078-T[14] Liu Y, Pharr M, Salvatore G A. Lab-on-skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano, 2017, 11(10), 9614 doi: 10.1021/acsnano.7b04898[15] Kim D H, Lu N, Ma R, et al. Epidermal electronics. Science, 2011, 333(6044), 838 doi: 10.1126/science.1206157[16] Bandodkar A J, Jeerapan I, You J M, et al. Highly stretchable fully-printed CNT-based electrochemical sensors and biofuel cells: combining intrinsic and design-induced stretchability. Nano Lett, 2016, 16(1), 721 doi: 10.1021/acs.nanolett.5b04549[17] Shyu T C, Damasceno P F, Dodd P M, et al. A kirigami approach to engineering elasticity in nanocomposites through patterned defects. Nat Mater, 2015, 14(8), 785 doi: 10.1038/nmat4327[18] Vandeparre H, Liu Q, Minev I R, et al. Localization of folds and cracks in thin metal films coated on flexible elastomer foams. Adv Mater, 2013, 25(22), 3117 doi: 10.1002/adma.201300587[19] Li K, Cheng X, Zhu F, et al. A generic soft encapsulation strategy for stretchable electronics. Adv Funct Mater, 2019, 29(8), 1806630 doi: 10.1002/adfm.201806630[20] Li J, Song E, Chiang C H, et al. Conductively coupled flexible silicon electronic systems for chronic neural electrophysiology. Proc Natl Acad Sci, 2018, 115(41), E9542 doi: 10.1073/pnas.1813187115[21] Cheng W, Yu L, Kong D, et al. Fast-response and low-hysteresis flexible pressure sensor based on silicon nanowires. IEEE Electron Device Lett, 2018, 39(7), 1069 doi: 10.1109/LED.2018.2835467[22] Wang Y, Zhu C, Pfattner R, et al. A highly stretchable, transparent, and conductive polymer. Sci Adv, 2017, 3(3), e1602076 doi: 10.1126/sciadv.1602076[23] Nur R, Matsuhisa N, Jiang Z, et al. A highly sensitive capacitive-type strain sensor using wrinkled ultrathin gold films. Nano Lett, 2018, 18(9), 5610 doi: 10.1021/acs.nanolett.8b02088[24] Müller C, Goffri S, Breiby D W, et al. Tough, semiconducting polyethylene-poly(3-hexylthiophene) diblock copolymers. Adv Funct Mater, 2007, 17(15), 2674 doi: 10.1002/adfm.200601248[25] O’Connor B, Kline R J, Conrad B R, et al. Anisotropic structure and sharge transport in highly strain-aligned regioregular poly(3-hexylthiophene). Adv Funct Mater, 2011, 21(19), 3697 doi: 10.1002/adfm.201100904[26] Wang G J N, Shaw L, Xu J, et al. Inducing elasticity through oligo-siloxane crosslinks for intrinsically stretchable semiconducting polymers. Adv Funct Mater, 2016, 26(40), 7254 doi: 10.1002/adfm.201602603[27] Oh J Y, Rondeau-Gagné S, Chiu Y C, et al. Intrinsically stretchable and healable semiconducting polymer for organic transistors. Nature, 2016, 539(7629), 411 doi: 10.1038/nature20102[28] Si L, Massa M V, Dalnoki-Veress K, et al. Chain entanglement in thin freestanding polymer films. Phys Rev Lett, 2005, 94(12), 127801 doi: 10.1103/PhysRevLett.94.127801[29] Xu J, Wang S, Wang G J N, et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science, 2017, 355(6320), 59 doi: 10.1126/science.aah4496[30] Kim Y, Zhu J, Yeom B, et al. Stretchable nanoparticle conductors with self-organized conductive pathways. Nature, 2013, 500(7460), 59 doi: 10.1038/nature12401[31] Trung T Q, Lee N E. Recent progress on stretchable electronic devices with intrinsically stretchable components. Adv Mater, 2017, 29(3), 1603167 doi: 10.1002/adma.201603167[32] Ma Z, Shi W, Yan K, et al. Doping engineering of conductive polymer hydrogels and their application in advanced sensor technologies. Chem Sci, 2019, 10(25), 6232 doi: 10.1039/C9SC02033K[33] Song E, Kang B, Choi H H, et al. Stretchable and transparent organic semiconducting thin film with conjugated polymer nanowires embedded in an elastomeric matrix. Adv Electron Mater, 2016, 2(1), 1500250 doi: 10.1002/aelm.201500250[34] Zhang Y, Sheehan C J, Zhai J, et al. Polymer-embedded carbon nanotube ribbons for stretchable conductors. Adv Mater, 2010, 22(28), 3027 doi: 10.1002/adma.200904426[35] Trung T Q, Ramasundaram S, Hwang B U, et al. An all-elastomeric transparent and stretchable temperature sensor for body-attachable wearable electronics. Adv Mater, 2016, 28(3), 502 doi: 10.1002/adma.201504441[36] Lipomi D J, Vosgueritchian M, Tee B C K, et al. Skin-like pressure and strain sensors based on transparent elastic films of carbon nanotubes. Nat Nanotechnol, 2011, 6(12), 788 doi: 10.1038/nnano.2011.184[37] Liang J, Li L, Chen D, et al. Intrinsically stretchable and transparent thin-film transistors based on printable silver nanowires, carbon nanotubes and an elastomeric dielectric. Nat Commun, 2015, 6, 7647 doi: 10.1038/ncomms8647[38] Chen S, Jiang K, Lou Z, et al. Recent developments in graphene-based tactile sensors and E-skins. Adv Mater Technol, 2018, 3(2), 1700248 doi: 10.1002/admt.201700248[39] Wang C, Wu H, Chen Z, et al. Self-healing chemistry enables the stable operation of silicon microparticle anodes for high-energy lithium-ion batteries. Nat Chem, 2013, 5(12), 1042 doi: 10.1038/nchem.1802[40] Bandodkar A J, Mohan V, López C S, et al. Self-healing inks for autonomous repair of printable electrochemical devices. Adv Electron Mater, 2015, 1(12), 1500289 doi: 10.1002/aelm.201500289[41] Wang T, Zhang Y, Liu Q, et al. A self-healable, highly stretchable, and solution processable conductive polymer composite for ultrasensitive strain and pressure sensing. Adv Funct Mater, 2018, 28(7), 1705551 doi: 10.1002/adfm.201705551[42] Pu W, Jiang F, Chen P, et al. A POSS based hydrogel with mechanical robustness, cohesiveness and a rapid self-healing ability by electrostatic interaction. Soft Matter, 2017, 13(34), 5645 doi: 10.1039/C7SM01492A[43] Nakahata M, Takashima Y, Harada A. Highly flexible, tough, and self-healing supramolecular polymeric materials using host–guest interaction. Macromol Rapid Commun, 2016, 37(1), 86 doi: 10.1002/marc.201500473[44] Kang J, Son D, Wang G J N, et al. Tough and water-insensitive self-healing elastomer for robust electronic skin. Adv Mater, 2018, 30(13), 1706846 doi: 10.1002/adma.201706846[45] Wang G J N, Gasperini A, Bao Z. Stretchable polymer semiconductors for plastic electronics. Adv Electron Mater, 2018, 4(2), 1700429 doi: 10.1002/aelm.201700429[46] Li C H, Wang C, Keplinger C, et al. A highly stretchable autonomous self-healing elastomer. Nat Chem, 2016, 8(6), 618 doi: 10.1038/nchem.2492[47] Cao Y, Tan Y J, Li S, et al. Self-healing electronic skins for aquatic environments. Nat Electron, 2019, 2(2), 75 doi: 10.1038/s41928-019-0206-5[48] Kang J, Tok J B H, Bao Z. Self-healing soft electronics. Nat Electron, 2019, 2(4), 144 doi: 10.1038/s41928-019-0235-0[49] Son D, Kang J, Vardoulis O, et al. An integrated self-healable electronic skin system fabricated via dynamic reconstruction of a nanostructured conducting network. Nat Nanotechnol, 2018, 13(11), 1057 doi: 10.1038/s41565-018-0244-6[50] Guo K, Zhang D L, Zhang X M, et al. Conductive elastomers with autonomic self-healing properties. Angew Chem Int Ed, 2015, 54(41), 12127 doi: 10.1002/anie.201505790[51] D’Elia E, Barg S, Ni N, et al. Self-healing graphene-based composites with sensing capabilities. Adv Mater, 2015, 27(32), 4788 doi: 10.1002/adma.201501653[52] Yan X, Liu Z, Zhang Q, et al. Quadruple H-bonding cross-linked supramolecular polymeric materials as substrates for stretchable, antitearing, and self-healable thin film electrodes. J Am Chem Soc, 2018, 140(15), 5280 doi: 10.1021/jacs.8b01682[53] Tee B C K, Wang C, Allen R, et al. An electrically and mechanically self-healing composite with pressure-and flexion-sensitive properties for electronic skin applications. Nat Nanotechnol, 2012, 7(12), 825 doi: 10.1038/nnano.2012.192[54] Khatib M, Huynh T P, Deng Y, et al. A freestanding stretchable and multifunctional transistor with intrinsic self-healing properties of all device components. Small, 2019, 15(2), 1803939 doi: 10.1002/smll.201803939[55] Dvir T, Timko B P, Brigham M D, et al. Nanowired three-dimensional cardiac patches. Nat Nanotechnol, 2011, 6(11), 720 doi: 10.1038/nnano.2011.160[56] Bettinger C J, Bao Z. Organic thin-film transistors fabricated on resorbable biomaterial substrates. Adv Mater, 2010, 22(5), 651 doi: 10.1002/adma.200902322[57] Salvatore G A, Sülzle J, Dalla Valle F, et al. Biodegradable and highly deformable temperature sensors for the internet of things. Adv Funct Mater, 2017, 27(35), 1702390 doi: 10.1002/adfm.201702390[58] Hwang S W, Park G, Cheng H, et al. Materials for high-performance biodegradable semiconductor devices. Adv Mater, 2014, 26(13), 1992 doi: 10.1002/adma.201304821[59] Lu D, Liu T L, Chang J K, et al. Transient light-emitting diodes constructed from semiconductors and transparent conductors that biodegrade under physiological conditions. Adv Mater, 2019, 31(42), 1902739 doi: 10.1002/adma.201902739[60] Hwang S W, Tao H, Kim D H, et al. A physically transient form of silicon electronics. Science, 2012, 337(6102), 1640 doi: 10.1126/science.1226325[61] Hwang S W, Lee C H, Cheng H, et al. Biodegradable elastomers and silicon nanomembranes/nanoribbons for stretchable, transient electronics, and biosensors. Nano Lett, 2015, 15(5), 2801 doi: 10.1021/nl503997m[62] Hwang S W, Huang X, Seo J H, et al. Materials for bioresorbable radio frequency electronics. Adv Mater, 2013, 25(26), 3526 doi: 10.1002/adma.201300920[63] Kang S K, Murphy R K J, Hwang S W, et al. Bioresorbable silicon electronic sensors for the brain. Nature, 2016, 530(7588), 71 doi: 10.1038/nature16492[64] Bai W, Shin J, Fu R, et al. Bioresorbable photonic devices for the spectroscopic characterization of physiological status and neural activity. Nat Biomed Eng, 2019, 3(8), 644 doi: 10.1038/s41551-019-0435-y[65] Shin J, Liu Z, Bai W, et al. Bioresorbable optical sensor systems for monitoring of intracranial pressure and temperature. Sci Adv, 2019, 5(7), eaaw1899 doi: 10.1126/sciadv.aaw1899[66] Lei T, Chen X, Pitner G, et al. Removable and recyclable conjugated polymers for highly selective and high-yield dispersion and release of low-cost carbon nanotubes. J Am Chem Soc, 2016, 138(3), 802 doi: 10.1021/jacs.5b12797[67] Irimia-Vladu M, Troshin P A, Reisinger M, et al. Biocompatible and biodegradable materials for organic field-effect transistors. Adv Funct Mater, 2010, 20(23), 4069 doi: 10.1002/adfm.201001031[68] Irimia-Vladu M, Głowacki E D, Troshin P A, et al. Indigo-a natural pigment for high performance ambipolar organic field effect transistors and circuits. Adv Mater, 2012, 24(3), 375 doi: 10.1002/adma.201102619[69] Wang L, Wang K, Lou Z, et al. Plant-based modular building blocks for “green” electronic skins. Adv Funct Mater, 2018, 28(51), 1804510 doi: 10.1002/adfm.201804510[70] Wang C, Li X, Gao E, et al. Carbonized silk fabric for ultrastretchable, highly sensitive, and wearable strain sensors. Adv Mater, 2016, 28(31), 6640 doi: 10.1002/adma.201601572[71] Wang C, Xia K, Zhang M, et al. An all-silk-derived dual-mode e-skin for simultaneous temperature–pressure detection. ACS Appl Mater Interfaces, 2017, 9(45), 39484 doi: 10.1021/acsami.7b13356[72] Boutry C M, Kaizawa Y, Schroeder B C, et al. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat Electron, 2018, 1(5), 314 doi: 10.1038/s41928-018-0071-7[73] Khodagholy D, Gelinas J N, Thesen T, et al. NeuroGrid: recording action potentials from the surface of the brain. Nat Neurosci, 2015, 18(2), 310 doi: 10.1038/nn.3905[74] Lei T, Guan M, Liu J, et al. Biocompatible and totally disintegrable semiconducting polymer for ultrathin and ultralightweight transient electronics. Proc Natl Acad Sci, 2017, 114(20), 5107 doi: 10.1073/pnas.1701478114[75] Boutry C M, Beker L, Kaizawa Y, et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat Biomed Eng, 2019, 3(1), 47 doi: 10.1038/s41551-018-0336-5[76] Mannsfeld S C B, Tee B C K, Stoltenberg R M, et al. Highly sensitive flexible pressure sensors with microstructured rubber dielectric layers. Nat Mater, 2010, 9(10), 859 doi: 10.1038/nmat2834[77] Boutry C M, Negre M, Jorda M, et al. A hierarchically patterned, bioinspired e-skin able to detect the direction of applied pressure for robotics. Sci Robot, 2018, 3(24), eaau6914 doi: 10.1126/scirobotics.aau6914[78] Low Z W K, Li Z, Owh C, et al. Using artificial skin devices as skin replacements: insights into superficial treatment. Small, 2019, 15(9), 1805453 doi: 10.1002/smll.201805453[79] Boutry C M, Nguyen A, Lawal Q O, et al. A sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv Mater, 2015, 27(43), 6954 doi: 10.1002/adma.201502535[80] Tee B C K, Chortos A, Dunn R R, et al. Tunable flexible pressure sensors using microstructured elastomer geometries for intuitive electronics. Adv Funct Mater, 2014, 24(34), 5427 doi: 10.1002/adfm.201400712[81] Bae G Y, Han J T, Lee G, et al. Pressure/temperature sensing bimodal electronic skin with stimulus discriminability and linear sensitivity. Adv Mater, 2018, 30(43), 1803388 doi: 10.1002/adma.201803388[82] Wang Z, Guo S, Li H, et al. The semiconductor/conductor interface piezoresistive effect in an organic transistor for highly sensitive pressure sensors. Adv Mater, 2019, 31(6), 1805630 doi: 10.1002/adma.201805630[83] Cho S H, Lee S W, Yu S, et al. Micropatterned pyramidal ionic gels for sensing broad-range pressures with high sensitivity. ACS Appl Mater Interfaces, 2017, 9(11), 10128 doi: 10.1021/acsami.7b00398[84] Schwartz G, Tee B C K, Mei J, et al. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat Commun, 2013, 4, 1859 doi: 10.1038/ncomms2832[85] Cheng W, Wang J, Ma Z, et al. Flexible pressure sensor with high sensitivity and low hysteresis based on a hierarchically microstructured electrode. IEEE Electron Device Lett, 2018, 39(2), 288 doi: 10.1109/LED.2017.2784538[86] Chou H H, Nguyen A, Chortos A, et al. A chameleon-inspired stretchable electronic skin with interactive colour changing controlled by tactile sensing. Nat Commun, 2015, 6, 8011 doi: 10.1038/ncomms9011[87] Pan L, Chortos A, Yu G, et al. An ultra-sensitive resistive pressure sensor based on hollow-sphere microstructure induced elasticity in conducting polymer film. Nat Commun, 2014, 5, 3002 doi: 10.1038/ncomms4002[88] Bae G Y, Pak S W, Kim D, et al. Linearly and highly pressure-sensitive electronic skin based on a bioinspired hierarchical structural array. Adv Mater, 2016, 28(26), 5300 doi: 10.1002/adma.201600408[89] Pang C, Lee G Y, Kim T, et al. A flexible and highly sensitive strain-gauge sensor using reversible interlocking of nanofibres. Nat Mater, 2012, 11(9), 795 doi: 10.1038/nmat3380[90] Kang D, Pikhitsa P V, Choi Y W, et al. Ultrasensitive mechanical crack-based sensor inspired by the spider sensory system. Nature, 2014, 516(7530), 222 doi: 10.1038/nature14002[91] Ranade S S, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron, 2015, 87(6), 1162 doi: 10.1016/j.neuron.2015.08.032[92] Schrenk-Siemens K, Wende H, Prato V, et al. PIEZO2 is required for mechanotransduction in human stem cell–derived touch receptors. Nat Neurosci, 2015, 18(1), 10 doi: 10.1038/nn.3894[93] Jin M L, Park S, Lee Y, et al. An ultrasensitive, visco-poroelastic artificial mechanotransducer skin inspired by piezo2 protein in mammalian merkel cells. Adv Mater, 2017, 29(13), 1605973 doi: 10.1002/adma.201605973[94] Lee C Y, Wu G W, Hsieh W J. Fabrication of micro sensors on a flexible substrate. Sens Actuators Phys, 2008, 147(1), 173 doi: 10.1016/j.sna.2008.05.004[95] Han I Y, Kim S J. Diode temperature sensor array for measuring micro-scale surface temperatures with high resolution. Sens Actuators Phys, 2008, 141(1), 52 doi: 10.1016/j.sna.2007.07.020[96] Webb R C, Bonifas A P, Behnaz A, et al. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat Mater, 2013, 12(10), 938 doi: 10.1038/nmat3755[97] Jeon J, Lee H B, Bao Z. Flexible wireless temperature sensors based on Ni microparticle-filled binary polymer composites. Adv Mater, 2013, 25(6), 850 doi: 10.1002/adma.201204082[98] Yeo W H, Kim Y S, Lee J, et al. Multifunctional epidermal electronics printed directly onto the skin. Adv Mater, 2013, 25(20), 2773 doi: 10.1002/adma.201204426[99] Chortos A, Bao Z. Skin-inspired electronic devices. Mater Today, 2014, 17(7), 321 doi: 10.1016/j.mattod.2014.05.006[100] Zhao S, Zhu R. Flexible bimodal sensor for simultaneous and independent perceiving of pressure and temperature stimuli. Adv Mater Technol, 2017, 2(11), 1700183 doi: 10.1002/admt.201700183[101] Ho D H, Sun Q, Kim S Y, et al. Stretchable and multimodal all graphene electronic skin. Adv Mater, 2016, 28(13), 2601 doi: 10.1002/adma.201505739[102] Wang Q, Jian M, Wang C, et al. Carbonized silk nanofiber membrane for transparent and sensitive electronic skin. Adv Funct Mater, 2017, 27(9), 1605657 doi: 10.1002/adfm.201605657[103] Gao W, Emaminejad S, Nyein H Y Y, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature, 2016, 529(7587), 509 doi: 10.1038/nature16521[104] Sempionatto R J, Nakagawa T, Pavinatto A, et al. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab Chip, 2017, 17(10), 1834 doi: 10.1039/C7LC00192D[105] Wang L, Lou Z, Jiang K, et al. Bio-multifunctional smart wearable sensors for medical devices. Adv Intell Syst, 2019, 0(0), 1900040 doi: 10.1002/aisy.201900040[106] Yokota T, Sekitani T, Tokuhara T, et al. Sheet-type flexible organic active matrix amplifier system using pseudo-CMOS circuits with floating-gate structure. IEEE Trans Electron Devices, 2012, 59(12), 3434 doi: 10.1109/TED.2012.2220853[107] Ishida K, Huang T, Honda K, et al. Insole pedometer with piezoelectric energy harvester and 2 V organic circuits. IEEE J Solid-State Circuits, 2013, 48(1), 255 doi: 10.1109/JSSC.2012.2221253[108] Ji X, Zhou P, Zhong L, et al. Smart surgical catheter for C-reactive protein sensing based on an imperceptible organic transistor. Adv Sci, 2018, 5(6), 1701053 doi: 10.1002/advs.201701053[109] Chortos A, Koleilat G I, Pfattner R, et al. Mechanically durable and highly stretchable transistors employing carbon nanotube semiconductor and electrodes. Adv Mater, 2016, 28(22), 4441 doi: 10.1002/adma.201501828[110] Viventi J, Kim D H, Vigeland L, et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat Neurosci, 2011, 14(12), 1599 doi: 10.1038/nn.2973[111] Kong D, Pfattner R, Chortos A, et al. Capacitance characterization of elastomeric dielectrics for applications in intrinsically stretchable thin film transistors. Adv Funct Mater, 2016, 26(26), 4680 doi: 10.1002/adfm.201600612[112] Chortos A, Lim J, To J W F, et al. Highly stretchable transistors using a microcracked organic semiconductor. Adv Mater, 2014, 26(25), 4253 doi: 10.1002/adma.201305462[113] Nawrocki R A, Matsuhisa N, Yokota T, et al. 300-nm imperceptible, ultraflexible, and biocompatible e-skin fit with tactile sensors and organic transistors. Adv Electron Mater, 2016, 2(4), 1500452 doi: 10.1002/aelm.201500452[114] Lee S, Reuveny A, Reeder J, et al. A transparent bending-insensitive pressure sensor. Nat Nanotechnol, 2016, 11(5), 472 doi: 10.1038/nnano.2015.324[115] Sun Q, Seung W, Kim B J, et al. Active matrix electronic skin strain sensor based on piezopotential-powered graphene transistors. Adv Mater, 2015, 27(22), 3411 doi: 10.1002/adma.201500582[116] Someya T, Sekitani T, Iba S, et al. A large-area, flexible pressure sensor matrix with organic field-effect transistors for artificial skin applications. Proc Natl Acad Sci, 2004, 101(27), 9966 doi: 10.1073/pnas.0401918101[117] Pfattner R, Foudeh A M, Liong C, et al. On the working mechanisms of solid-state double-layer-dielectric-based organic field-effect transistors and their implication for sensors. Adv Electron Mater, 2018, 4(1), 1700326 doi: 10.1002/aelm.201700326[118] Pfattner R, Foudeh A M, Chen S, et al. Dual-gate organic field-effect transistor for pH sensors with tunable sensitivity. Adv Electron Mater, 2019, 5(1), 1800381 doi: 10.1002/aelm.201800381[119] Zhu C, Wu H C, Nyikayaramba G, et al. Intrinsically stretchable temperature sensor based on organic thin-film transistors. IEEE Electron Device Lett, 2019, 40(10), 1630 doi: 10.1109/LED.2019.2933838[120] Sun Q, Kim D H, Park S S, et al. Transparent, low-power pressure sensor matrix based on coplanar-gate graphene transistors. Adv Mater, 2014, 26(27), 4735 doi: 10.1002/adma.201400918[121] Sekitani T, Yokota T, Kuribara K, et al. Ultraflexible organic amplifier with biocompatible gel electrodes. Nat Commun, 2016, 7(1), 1 doi: 10.1038/ncomms11425[122] Reuveny A, Lee S, Yokota T, et al. High-frequency, conformable organic amplifiers. Adv Mater, 2016, 28(17), 3298 doi: 10.1002/adma.201505381[123] Matsuhisa N, Jiang Y, Liu Z, et al. High-transconductance stretchable transistors achieved by controlled gold microcrack morphology. Adv Electron Mater, 2019, 5(8), 1900347 doi: 10.1002/aelm.201900347[124] Molina-Lopez F, Gao T Z, Kraft U, et al. Inkjet-printed stretchable and low voltage synaptic transistor array. Nat Commun, 2019, 10(1), 1 doi: 10.1038/s41467-018-07882-8[125] Wang S, Xu J, Wang W, et al. Skin electronics from scalable fabrication of an intrinsically stretchable transistor array. Nature, 2018, 555(7694), 83 doi: 10.1038/nature25494[126] Sugiyama M, Uemura T, Kondo M, et al. An ultraflexible organic differential amplifier for recording electrocardiograms. Nat Electron, 2019, 2(8), 351 doi: 10.1038/s41928-019-0283-5[127] Lee H, Lee S, Lee W, et al. Ultrathin organic electrochemical transistor with nonvolatile and thin gel electrolyte for long-term electrophysiological monitoring. Adv Funct Mater, 2019, 1906982 doi: 10.1002/adfm.201906982[128] Uz M, Jackson K, Donta M S, et al. Fabrication of high-resolution graphene-based flexible electronics via polymer casting. Sci Rep, 2019, 9(1), 10595 doi: 10.1038/s41598-019-46978-z[129] Liu S C, Delbruck T. Neuromorphic sensory systems. Curr Opin Neurobiol, 2010, 20(3), 288 doi: 10.1016/j.conb.2010.03.007[130] Zhu C, Chortos A, Wang Y, et al. Stretchable temperature-sensing circuits with strain suppression based on carbon nanotube transistors. Nat Electron, 2018, 1(3), 183 doi: 10.1038/s41928-018-0041-0[131] Kyaw A K K, Loh H H C, Yan F, et al. A polymer transistor array with a pressure-sensitive elastomer for electronic skin. J Mater Chem C, 2017, 5(46), 12039 doi: 10.1039/C7TC03293E[132] Kim J, Son D, Lee M, et al. A wearable multiplexed silicon nonvolatile memory array using nanocrystal charge confinement. Sci Adv, 2016, 2(1), e1501101 doi: 10.1126/sciadv.1501101[133] Wang H, Wei P, Li Y, et al. Tuning the threshold voltage of carbon nanotube transistors by n-type molecular doping for robust and flexible complementary circuits. Proc Natl Acad Sci, 2014, 111(13), 4776 doi: 10.1073/pnas.1320045111[134] Kim J, Banks A, Cheng H, et al. Epidermal electronics with advanced capabilities in near-field communication. Small, 2015, 11(8), 906 doi: 10.1002/smll.201402495[135] Kim J, Banks A, Xie Z, et al. Miniaturized flexible electronic systems with wireless power and near-field communication capabilities. Adv Funct Mater, 2015, 25(30), 4761 doi: 10.1002/adfm.201501590[136] Miyamoto A, Lee S, Cooray N F, et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat Nanotechnol, 2017, 12(9), 907 doi: 10.1038/nnano.2017.125[137] Tian L, Zimmerman B, Akhtar A, et al. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat Biomed Eng, 2019, 3(3), 194 doi: 10.1038/s41551-019-0347-x[138] Zhang Y, Fu H, Xu S, et al. A hierarchical computational model for stretchable interconnects with fractal-inspired designs. J Mech Phys Solids, 2014, 72, 115 doi: 10.1016/j.jmps.2014.07.011[139] Huang Z, Hao Y, Li Y, et al. Three-dimensional integrated stretchable electronics. Nat Electron, 2018, 1(8), 473 doi: 10.1038/s41928-018-0116-y[140] Xu B, Akhtar A, Liu Y, et al. An epidermal stimulation and sensing platform for sensorimotor prosthetic control, management of lower back exertion, and electrical muscle activation. Adv Mater, 2016, 28(22), 4462 doi: 10.1002/adma.201504155[141] Chen H, Cao Y, Zhang J, et al. Large-scale complementary macroelectronics using hybrid integration of carbon nanotubes and IGZO thin-film transistors. Nat Commun, 2014, 5, 4097 doi: 10.1038/ncomms5097[142] Lee H E, Kim S, Ko J, et al. Skin-like oxide thin-film transistors for transparent displays. Adv Funct Mater, 2016, 26(34), 6170 doi: 10.1002/adfm.201601296[143] Lei T, Shao L L, Zheng Y Q, et al. Low-voltage high-performance flexible digital and analog circuits based on ultrahigh-purity semiconducting carbon nanotubes. Nat Commun, 2019, 10(1), 2161 doi: 10.1038/s41467-019-10145-9[144] Tee B C K, Chortos A, Berndt A, et al. A skin-inspired organic digital mechanoreceptor. Science, 2015, 350(6258), 313 doi: 10.1126/science.aaa9306[145] Yeom C, Chen K, Kiriya D, et al. Large-area compliant tactile sensors using printed carbon nanotube active-matrix backplanes. Adv Mater, 2015, 27(9), 1561 doi: 10.1002/adma.201404850[146] Sekitani T, Yokota T, Zschieschang U, et al. Organic nonvolatile memory transistors for flexible sensor arrays. Science, 2009, 326(5959), 1516 doi: 10.1126/science.1179963[147] Bartolozzi C, Natale L, Nori F, et al. Robots with a sense of touch. Nat Mater, 2016, 15, 921 doi: 10.1038/nmat4731[148] Kim J, Sempionatto J R, Imani S, et al. Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform. Adv Sci, 2018, 5(10), 1800880 doi: 10.1002/advs.201800880[149] Lee H, Song C, Baik S, et al. Device-assisted transdermal drug delivery. Adv Drug Deliv Rev, 2018, 127, 35 doi: 10.1016/j.addr.2017.08.009[150] Chung H U, Kim B H, Lee J Y, et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science, 2019, 363(6430), eaau0780 doi: 10.1126/science.aau0780[151] Ma Z, Chen P, Cheng W, et al. Highly sensitive, printable nanostructured conductive polymer wireless sensor for food spoilage detection. Nano Lett, 2018, 18(7), 4570 doi: 10.1021/acs.nanolett.8b01825[152] Bandodkar A J, Gutruf P, Choi J, et al. Battery-free, skin-interfaced microfluidic/electronic systems for simultaneous electrochemical, colorimetric, and volumetric analysis of sweat. Sci Adv, 2019, 5(1), eaav3294 doi: 10.1126/sciadv.aav3294[153] Cao Z, Chen P, Ma Z, et al. Near-field communication sensors. Sensors, 2019, 19(18), 3947 doi: 10.3390/s19183947[154] Chen L Y, Tee B C K, Chortos A L, et al. Continuous wireless pressure monitoring and mapping with ultra-small passive sensors for health monitoring and critical care. Nat Commun, 2014, 5, 5028 doi: 10.1038/ncomms6028[155] Niu S, Matsuhisa N, Beker L, et al. A wireless body area sensor network based on stretchable passive tags. Nat Electron, 2019, 2(8), 361 doi: 10.1038/s41928-019-0286-2[156] Maiolino P, Maggiali M, Cannata G, et al. A flexible and robust large scale capacitive tactile system for robots. IEEE Sens J, 2013, 13(10), 3910 doi: 10.1109/JSEN.2013.2258149[157] Liu M, Pu X, Jiang C, et al. Large-area all-textile pressure sensors for monitoring human motion and physiological signals. Adv Mater, 2017, 29(41), 1703700 doi: 10.1002/adma.201703700[158] Wu X, Han Y, Zhang X, et al. Large-area compliant, low-cost, and versatile pressure-sensing platform based on microcrack-designed carbon black@polyurethane sponge for human−machine interfacing. Adv Funct Mater, 2016, 26(34), 6246 doi: 10.1002/adfm.201601995[159] Kim J, Lee M, Shim H J, et al. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat Commun, 2014, 5, 5747 doi: 10.1038/ncomms6747[160] Viventi J, Kim D H, Moss J D, et al. A conformal, bio-interfaced class of silicon electronics for mapping cardiac electrophysiology. Sci Trans Med, 2010, 2(24), 24ra22 doi: 10.1126/scitranslmed.3000738[161] Park Y J, Sharma B K, Shinde S M, et al. All MoS2-based large area, skin-attachable active-matrix tactile sensor. ACS Nano, 2019, 13(3), 3023 doi: 10.1021/acsnano.8b07995[162] Kim S K, Kirchner E A, Stefes A, et al. Intrinsic interactive reinforcement learning – Using error-related potentials for real world human-robot interaction. Sci Rep, 2017, 7(1), 1 doi: 10.1038/s41598-016-0028-x[163] Sundaram S, Kellnhofer P, Li Y, et al. Learning the signatures of the human grasp using a scalable tactile glove. Nature, 2019, 569(7758), 698 doi: 10.1038/s41586-019-1234-z[164] Markovic M, Schweisfurth M A, Engels L F, et al. Myocontrol is closed-loop control: incidental feedback is sufficient for scaling the prosthesis force in routine grasping. J NeuroEngineering Rehabil, 2018, 15(1), 81 doi: 10.1186/s12984-018-0422-7[165] Gerratt A P, Michaud H O, Lacour S P. Elastomeric electronic skin for prosthetic tactile sensation. Adv Funct Mater, 2015, 25(15), 2287 doi: 10.1002/adfm.201404365[166] Jin X, Zhu D D, Chen B Z, et al. Insulin delivery systems combined with microneedle technology. Adv Drug Deliv Rev, 2018, 127, 119 doi: 10.1016/j.addr.2018.03.011[167] Tan E K W, Au Y Z, Moghaddam G K, et al. Towards closed-loop integration of point-of-care technologies. Trends Biotechnol, 2019, 37(7), 775 doi: 10.1016/j.tibtech.2018.12.004[168] Lee H, Choi T K, Lee Y B, et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat Nanotechnol, 2016, 11(6), 566 doi: 10.1038/nnano.2016.38[169] Zhang Y, Wang J, Yu J, et al. Bioresponsive microneedles with a sheath structure for H2O2 and pH cascade-triggered insulin delivery. Small, 2018, 14(14), 1704181 doi: 10.1002/smll.201704181[170] Lee H, Song C, Hong Y S, et al. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci Adv, 2017, 3(3), e1601314 doi: 10.1126/sciadv.1601314[171] Tong Z, Zhou J, Zhong J, et al. Glucose-and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats. ACS Appl Mater Interfaces, 2018, 10(23), 20014 doi: 10.1021/acsami.8b04484[172] Koh A, Kang D, Xue Y, et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci Trans Med, 2016, 8(366), 366ra165 doi: 10.1126/scitranslmed.aaf2593[173] Yu J, Zhang Y, Ye Y, et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci, 2015, 112(27), 8260 doi: 10.1073/pnas.1505405112[174] Minev I R, Musienko P, Hirsch A, et al. Electronic dura mater for long-term multimodal neural interfaces. Science, 2015, 347(6218), 159 doi: 10.1126/science.1260318[175] Li L, Pan L, Ma Z, et al. All inkjet-printed amperometric multiplexed biosensors based on nanostructured conductive hydrogel electrodes. Nano Lett, 2018, 18(6), 3322 doi: 10.1021/acs.nanolett.8b00003[176] Fu S, Tao J, Wu W, et al. Fabrication of large-area bimodal sensors by all-inkjet-printing. Adv Mater Technol, 2019, 4(4), 1800703 doi: 10.1002/admt.201800703 -
Proportional views





 DownLoad:
DownLoad: