| Citation: |
Wenzhe Li, Naveed Ur Rahman, Yeming Xian, Hang Yin, Yunkai Bao, Yi Long, Songyang Yuan, Yangyi Zhang, Yaxuan Yuan, Jiandong Fan. Regulation of the order–disorder phase transition in a Cs2NaFeCl6 double perovskite towards reversible thermochromic application[J]. Journal of Semiconductors, 2021, 42(7): 072202. doi: 10.1088/1674-4926/42/7/072202
****
W Z Li, N U Rahman, Y M Xian, H Yin, Y K Bao, Y Long, S Y Yuan, Y Y Zhang, Y X Yuan, J D Fan, Regulation of the order–disorder phase transition in a Cs2NaFeCl6 double perovskite towards reversible thermochromic application[J]. J. Semicond., 2021, 42(7): 072202. doi: 10.1088/1674-4926/42/7/072202.
|
Regulation of the order–disorder phase transition in a Cs2NaFeCl6 double perovskite towards reversible thermochromic application
DOI: 10.1088/1674-4926/42/7/072202
More Information
-
Abstract
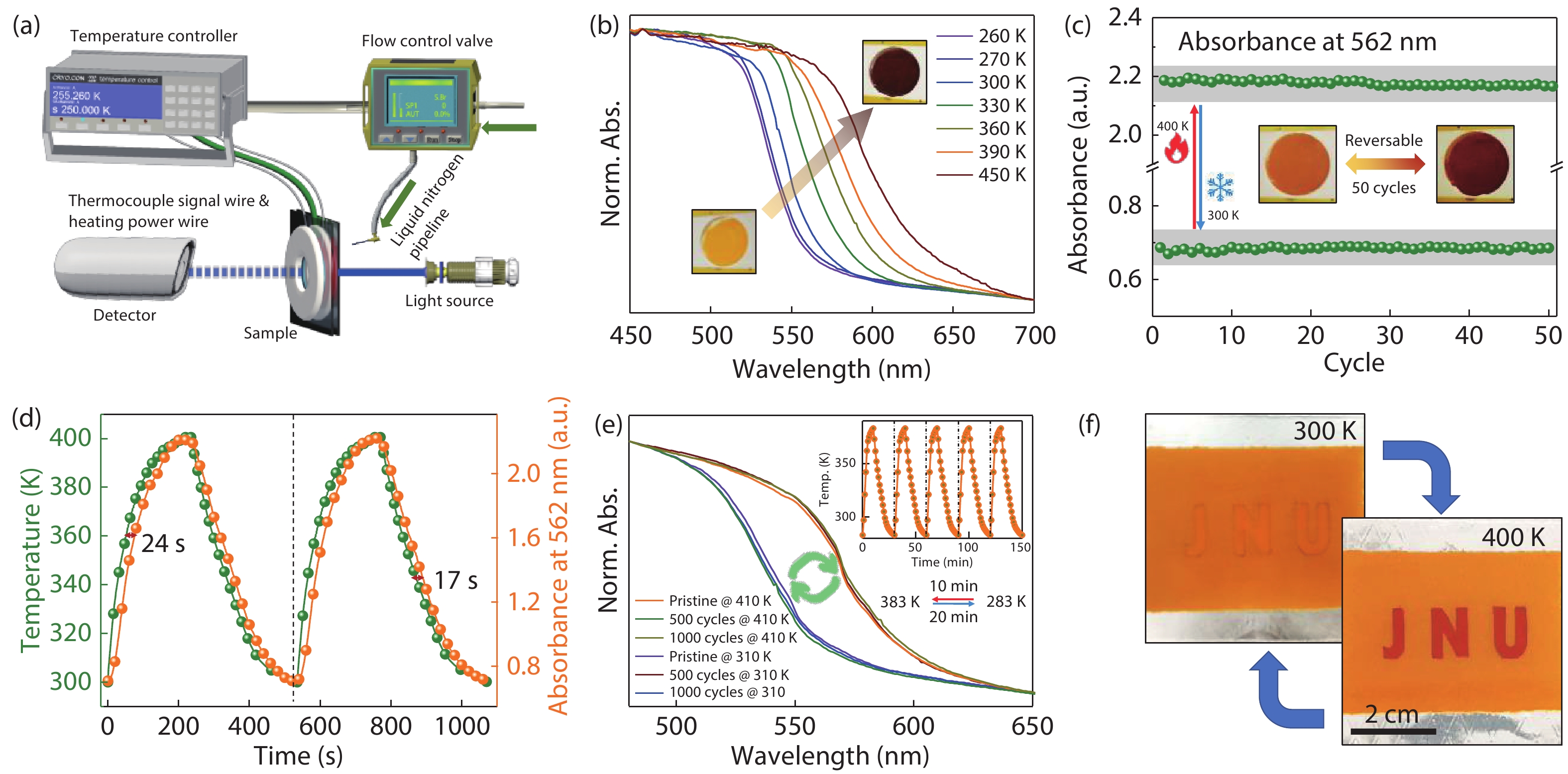
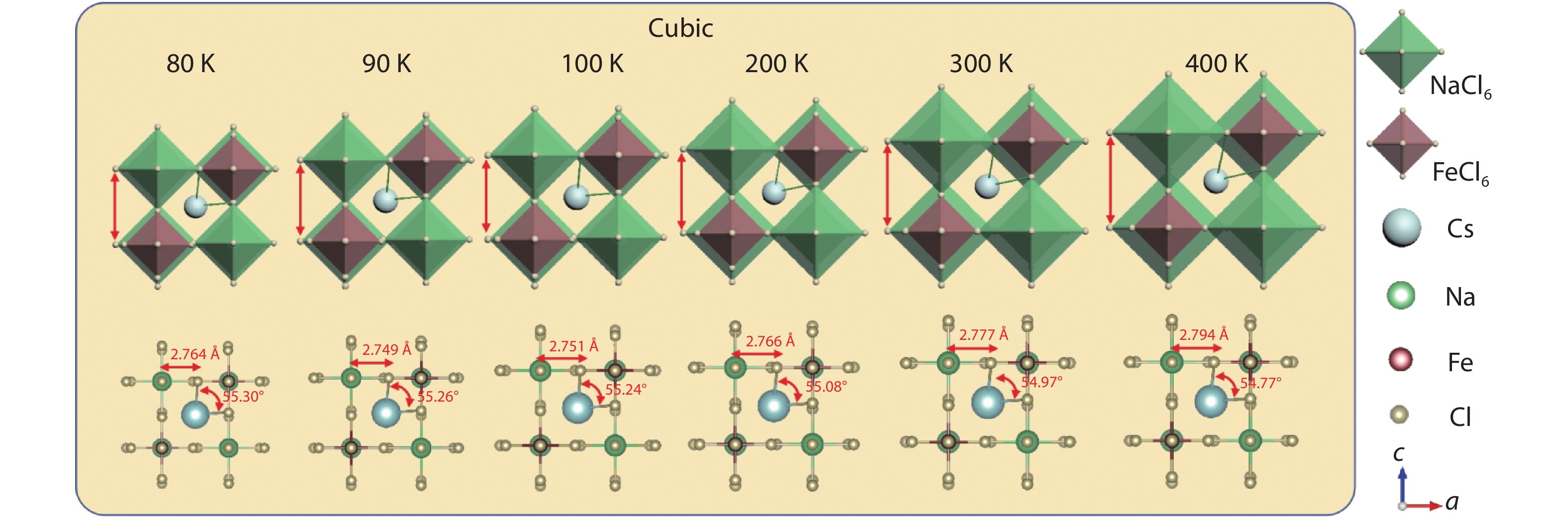
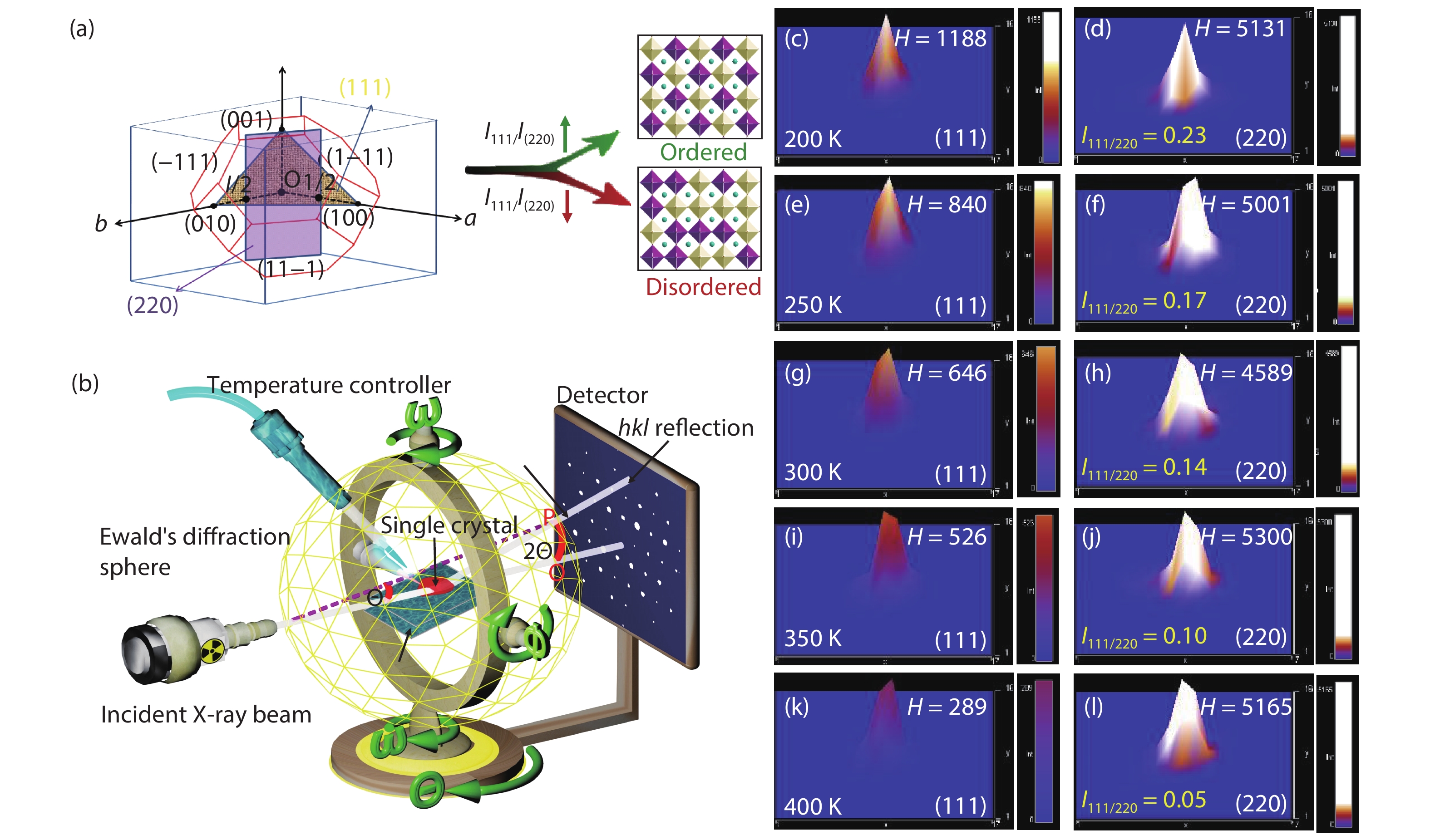
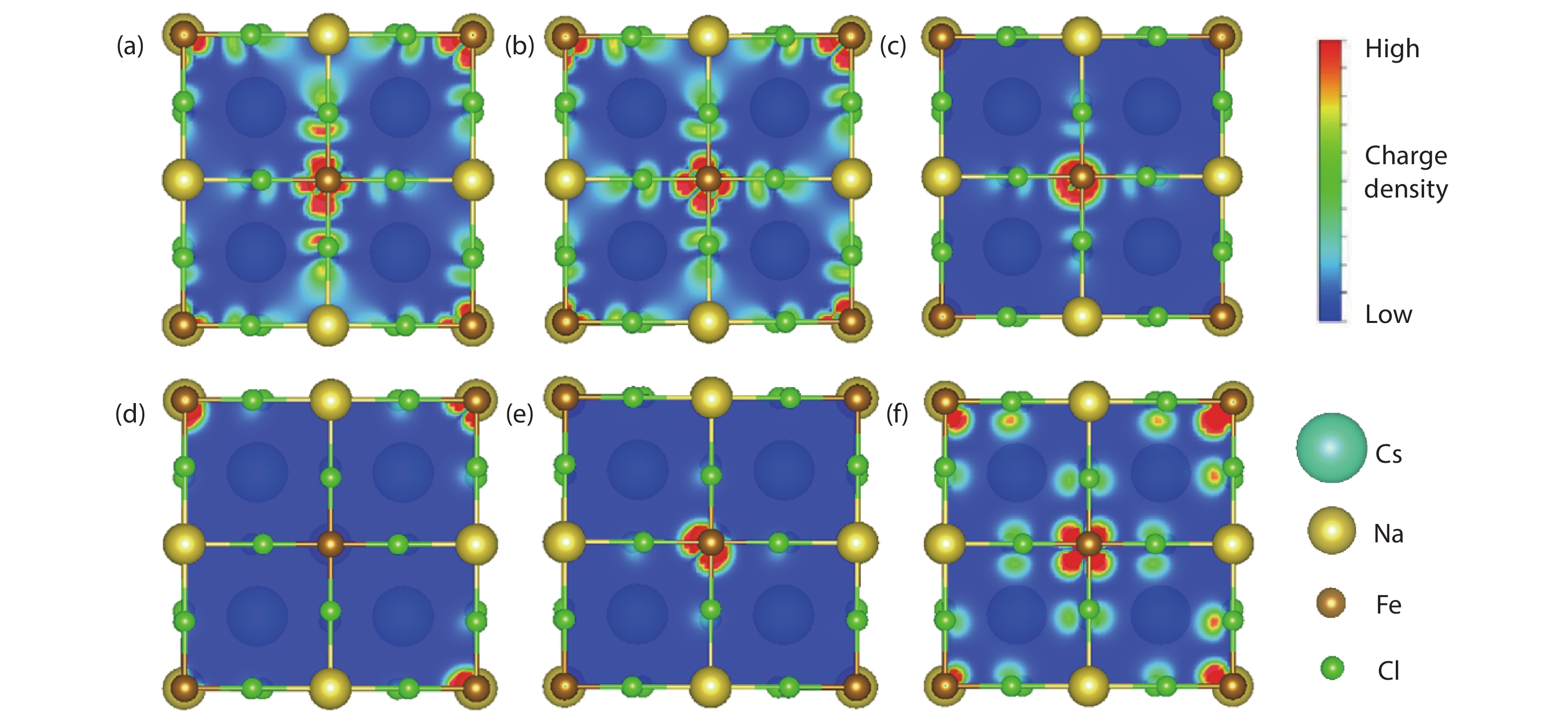
Multifunctional lead-free double perovskites demonstrate remarkable potential towards applications in various fields. Herein, an environmentally-friendly, low-cost, high-throughput Cs2NaFeCl6 single crystal with exceedingly high thermal stability is designed and grown. It obtains a cubic lattice system in the temperature range of 80–500 K, accompanied by a completely reversible chromatic variation ranging from yellow to black. Importantly, the intriguing thermochromism is proved to own extremely high reproducibility (over 1000 cycles) without a hysteretic effect, originating from its structural flexibility that including (i) the noteworthy distortion/deformation of [NaCl6]5− and [FeCl6]3− octahedra; (ii) order–disorder arrangement transition of [NaCl6]5− and [FeCl6]3− octahedra as the function of temperature. This study paves the way towards a new class of smart windows and camouflage coatings with an unprecedented colour range based on a Cs2NaFeCl6 perovskite. -
References
[1] Tsai H, Asadpour R, Blancon J C, et al. Light-induced lattice expansion leads to high-efficiency perovskite solar cells. Science, 2018, 360, 67 doi: 10.1126/science.aap8671[2] Hu M Y, Chen M, Guo P J, et al. Sub-1.4eV bandgap inorganic perovskite solar cells with long-term stability. Nat Commun, 2020, 11, 151 doi: 10.1038/s41467-019-13908-6[3] Shi E, Yuan B, Shiring S B, et al. Two-dimensional halide perovskite lateral epitaxial heterostructures. Nature, 2020, 580, 614 doi: 10.1038/s41586-020-2219-7[4] Park N G, Grätzel M, Miyasaka T, et al. Towards stable and commercially available perovskite solar cells. Nat Energy, 2016, 1, 16152 doi: 10.1038/nenergy.2016.152[5] Saliba M, Matsui T, Domanski K, et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science, 2016, 354, 206 doi: 10.1126/science.aah5557[6] Correa-Baena J P, Abate A, Saliba M, et al. The rapid evolution of highly efficient perovskite solar cells. Energy Environ Sci, 2017, 10, 710 doi: 10.1039/C6EE03397K[7] Lin J, Lai M L, Dou L T, et al. Thermochromic halide perovskite solar cells. Nat Mater, 2018, 17, 261 doi: 10.1038/s41563-017-0006-0[8] Pan W C, Wu H D, Luo J J, et al. Cs2AgBiBr6 single-crystal X-ray detectors with a low detection limit. Nat Photonics, 2017, 11, 726 doi: 10.1038/s41566-017-0012-4[9] Luo J J, Wang X M, Li S R, et al. Efficient and stable emission of warm-white light from lead-free halide double perovskites. Nature, 2018, 563, 541 doi: 10.1038/s41586-018-0691-0[10] Zhang Y, Tso C Y, Iñigo J S, et al. Perovskite thermochromic smart window: Advanced optical properties and low transition temperature. Appl Energy, 2019, 254, 113690 doi: 10.1016/j.apenergy.2019.113690[11] Ning W H, Zhao X G, Klarbring J, et al. Thermochromic lead-free halide double perovskites. Adv Funct Mater, 2019, 29, 1807375 doi: 10.1002/adfm.201807375[12] Halder A, Choudhury D, Ghosh S, et al. Exploring thermochromic behavior of hydrated hybrid perovskites in solar cells. J Phys Chem Lett, 2015, 6, 3180 doi: 10.1021/acs.jpclett.5b01426[13] Yuan W N, Niu G D, Xian Y M, et al. In situ regulating the order-disorder phase transition in Cs2AgBiBr6 single crystal toward the application in an X-ray detector. Adv Funct Mater, 2019, 29, 1900234 doi: 10.1002/adfm.201900234[14] Esser B, Hauser A, Williams R, et al. Quantitative STEM imaging of order-disorder phenomena in double perovskite thin films. Phys Rev Lett, 2016, 117, 176101 doi: 10.1103/PhysRevLett.117.176101[15] Yang J X, Zhang P, Wei S H. Band structure engineering of Cs2AgBiBr6 perovskite through order-disordered transition: A first-principle study. J Phys Chem Lett, 2018, 9, 31 doi: 10.1021/acs.jpclett.7b02992[16] Sheldrick G. SHELXL-97: crystal structure refinement program. University of Göttingen, Germany Göttingen, 1997[17] Sheldrick G. SHELXTL, structure determination software suite. Version 6.14, Bruker AXS, Madison Google Scholar, 2000[18] Dolomanov O V, Bourhis L J, Gildea R J, et al. OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr, 2009, 42, 339 doi: 10.1107/S0021889808042726[19] Osherov A, Hutter E M, Galkowski K, et al. The impact of phase retention on the structural and optoelectronic properties of metal halide perovskites. Adv Mater, 2016, 28, 10757 doi: 10.1002/adma.201604019[20] Han Q F, Bae S H, Sun P Y, et al. Single crystal formamidinium lead iodide (FAPbI3): Insight into the structural, optical, and electrical properties. Adv Mater, 2016, 28, 2253 doi: 10.1002/adma.201505002[21] Baikie T, Fang Y N, Kadro J M, et al. Synthesis and crystal chemistry of the hybrid perovskite (CH3NH3)PbI3 for solid-state sensitised solar cell applications. J Mater Chem A, 2013, 1, 5628 doi: 10.1039/c3ta10518k[22] Fan J D, Zhang H J, Wang J Y, et al. Growth and thermal properties of SrWO4 single crystal. J Appl Phys, 2006, 100, 063513 doi: 10.1063/1.2335510[23] Li L N, Sun Z H, Ji C M, et al. Rational design and syntheses of molecular phase transition crystal materials. Cryst Growth Des, 2016, 16, 6685 doi: 10.1021/acs.cgd.6b01378[24] Zunger A, Wei S H, Ferreira L G, et al. Special quasirandom structures. Phys Rev Lett, 1990, 65, 353 doi: 10.1103/PhysRevLett.65.353[25] Zhang W C, Sun Z H, Zhang J, et al. Thermochromism to tune the optical bandgap of a lead-free perovskite-type hybrid semiconductor for efficiently enhancing photocurrent generation. J Mater Chem C, 2017, 5, 9967 doi: 10.1039/C7TC02721D[26] Xiao Z W, Meng W W, Wang J B, et al. Thermodynamic stability and defect chemistry of bismuth-based lead-free double perovskites. ChemSusChem, 2016, 9, 2628 doi: 10.1002/cssc.201600771[27] Vineyard G H. Theory of order-disorder kinetics. Phys Rev, 1956, 102, 981 doi: 10.1103/PhysRev.102.981[28] Rey G, Redinger A, Sendler J, et al. The band gap of Cu2ZnSnSe4: Effect of order-disorder. Appl Phys Lett, 2014, 105, 112106 doi: 10.1063/1.4896315[29] Lim T W, Kim S D, Sung K D, et al. Insights into cationic ordering in Re-based double perovskite oxides. Sci Rep, 2016, 6, 19746 doi: 10.1038/srep19746[30] Setter N, Cross L E. The contribution of structural disorder to diffuse phase transitions in ferroelectrics. J Mater Sci, 1980, 15, 2478 doi: 10.1007/BF00550750[31] Yin H, Xian Y M, Zhang Y L, et al. Structurally stabilizing and environment friendly triggers: Double-metallic lead-free perovskites. Sol RRL, 2019, 3, 1900148 doi: 10.1002/solr.201900148[32] Wei S H, Ferreira L G, Bernard J E, et al. Electronic properties of random alloys: Special quasirandom structures. Phys Rev B, 1990, 42, 9622 doi: 10.1103/PhysRevB.42.9622 -
Supplements
 20120023suppl.pdf
20120023suppl.pdf

-
Proportional views






 DownLoad:
DownLoad: