| Citation: |
Li Wang, Yufeng Han, Hongchen Wang, Yaojie Han, Jinhua Liu, Gang Lu, Haidong Yu. A MXene-functionalized paper-based electrochemical immunosensor for label-free detection of cardiac troponin I[J]. Journal of Semiconductors, 2021, 42(9): 092601. doi: 10.1088/1674-4926/42/9/092601
****
L Wang, Y F Han, H C Wang, Y J Han, J H Liu, G Lu, H D Yu, A MXene-functionalized paper-based electrochemical immunosensor for label-free detection of cardiac troponin I[J]. J. Semicond., 2021, 42(9): 092601. doi: 10.1088/1674-4926/42/9/092601.
|
A MXene-functionalized paper-based electrochemical immunosensor for label-free detection of cardiac troponin I
DOI: 10.1088/1674-4926/42/9/092601
More Information
-
Abstract
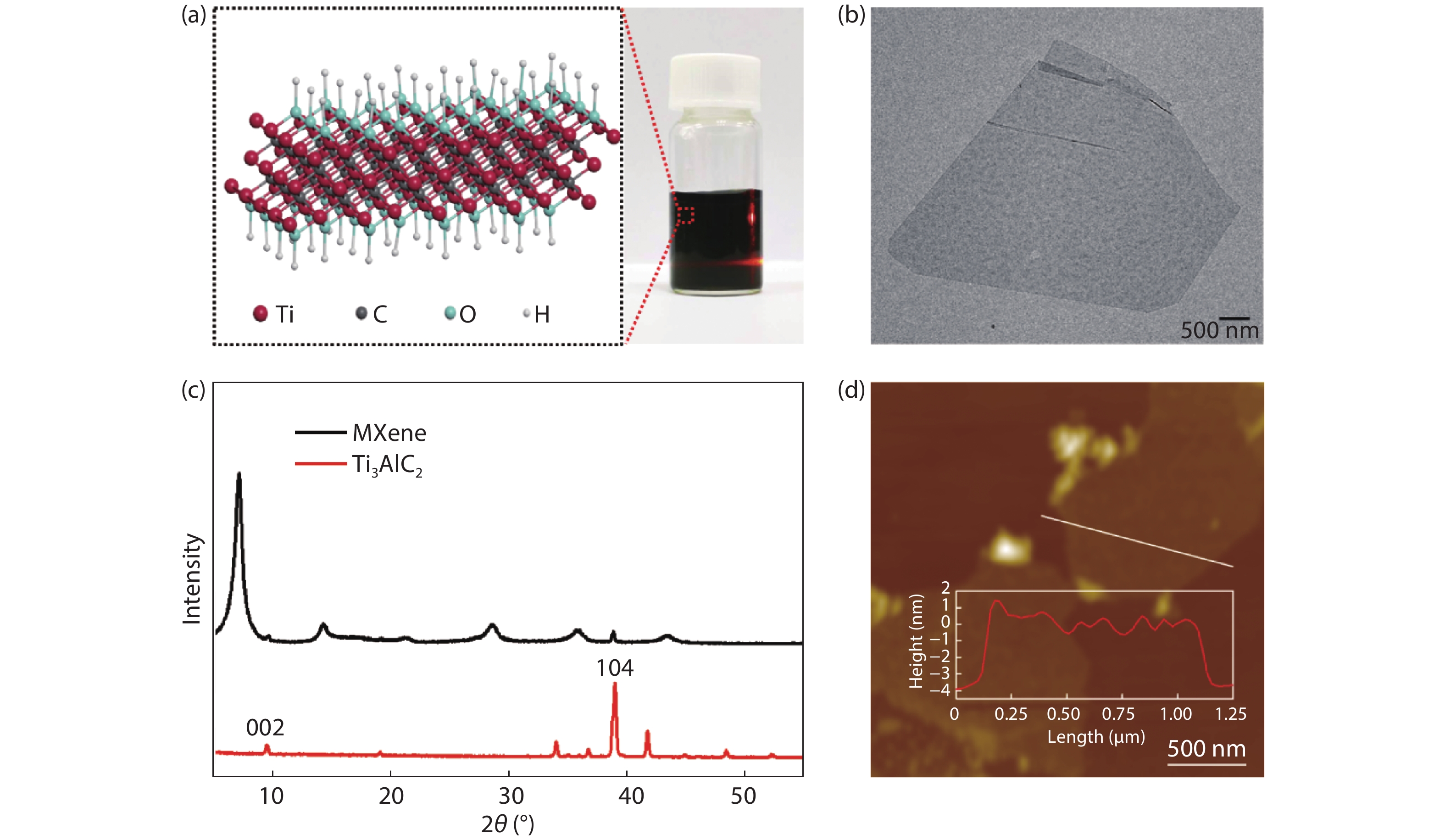
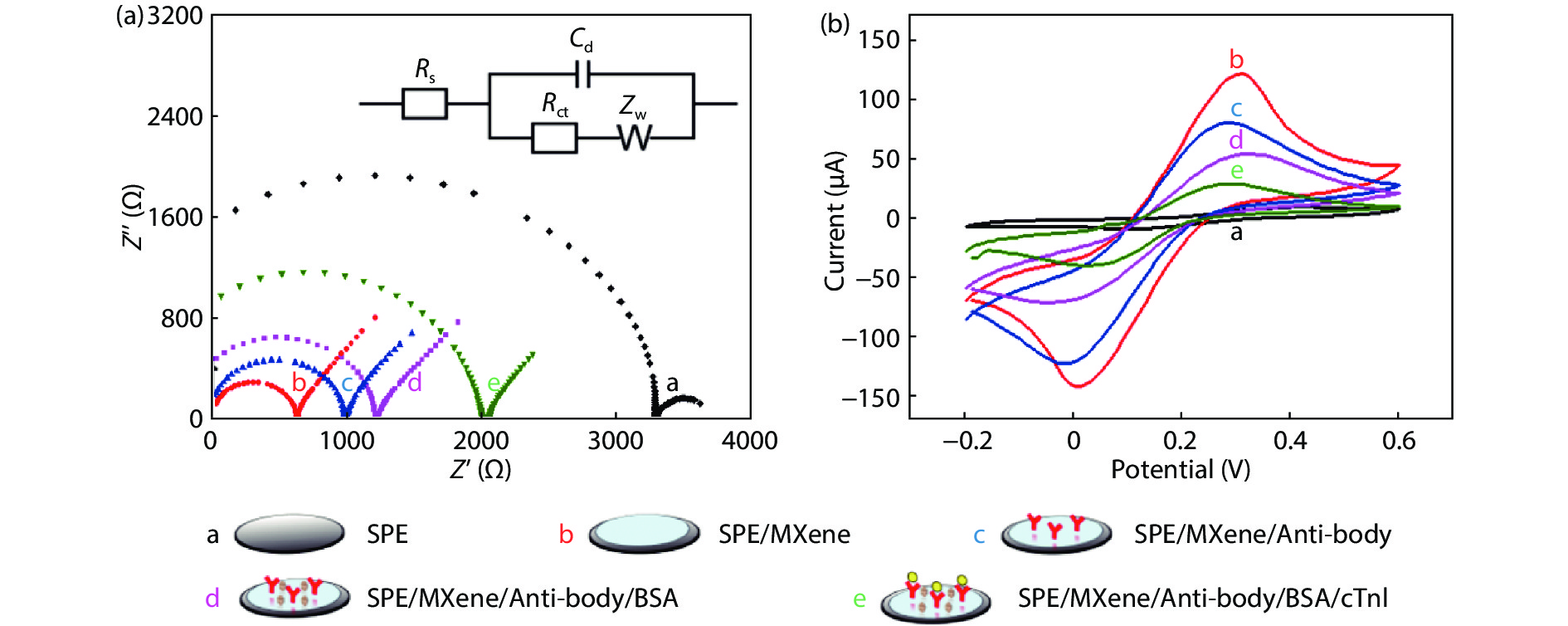
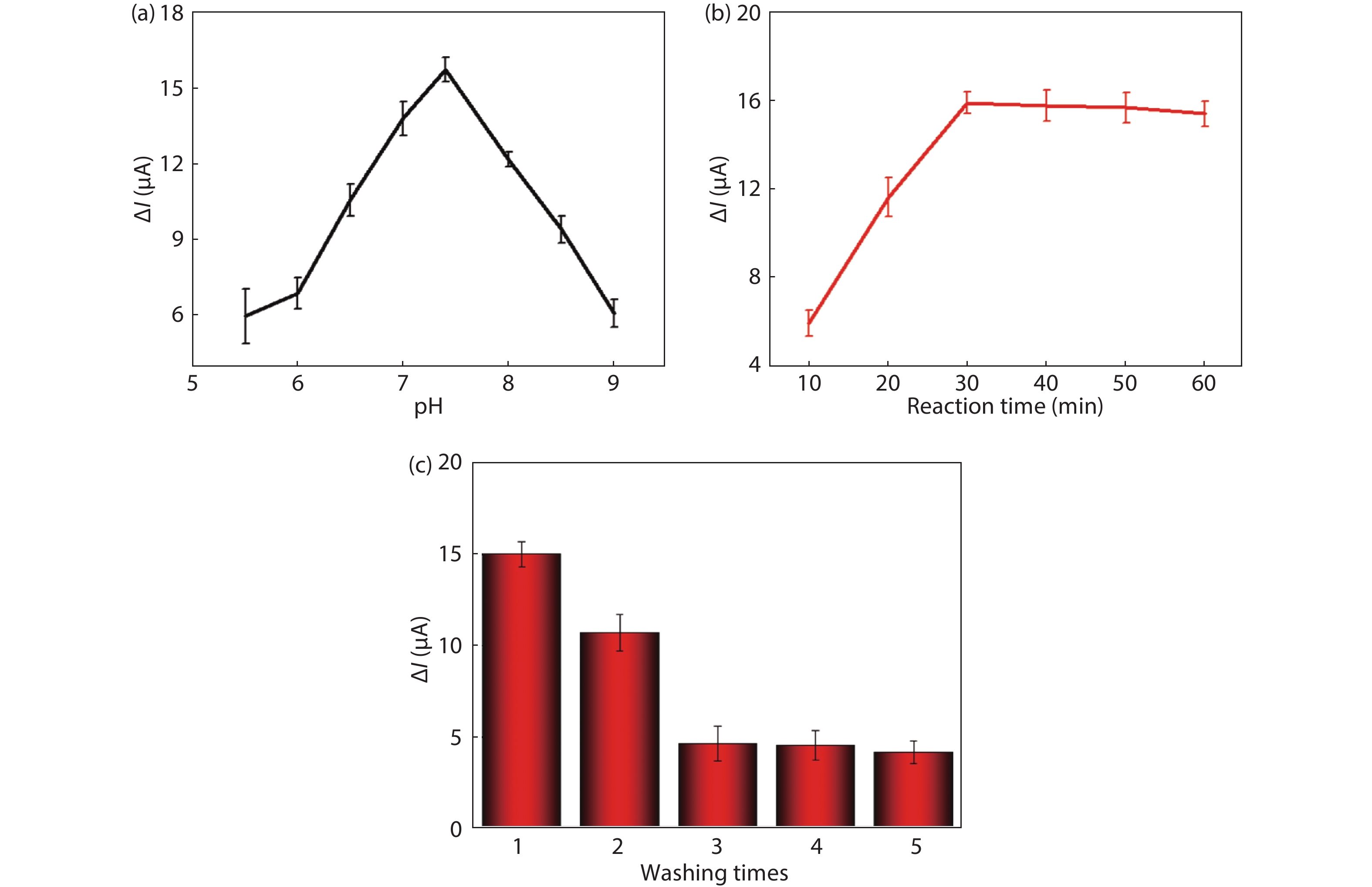
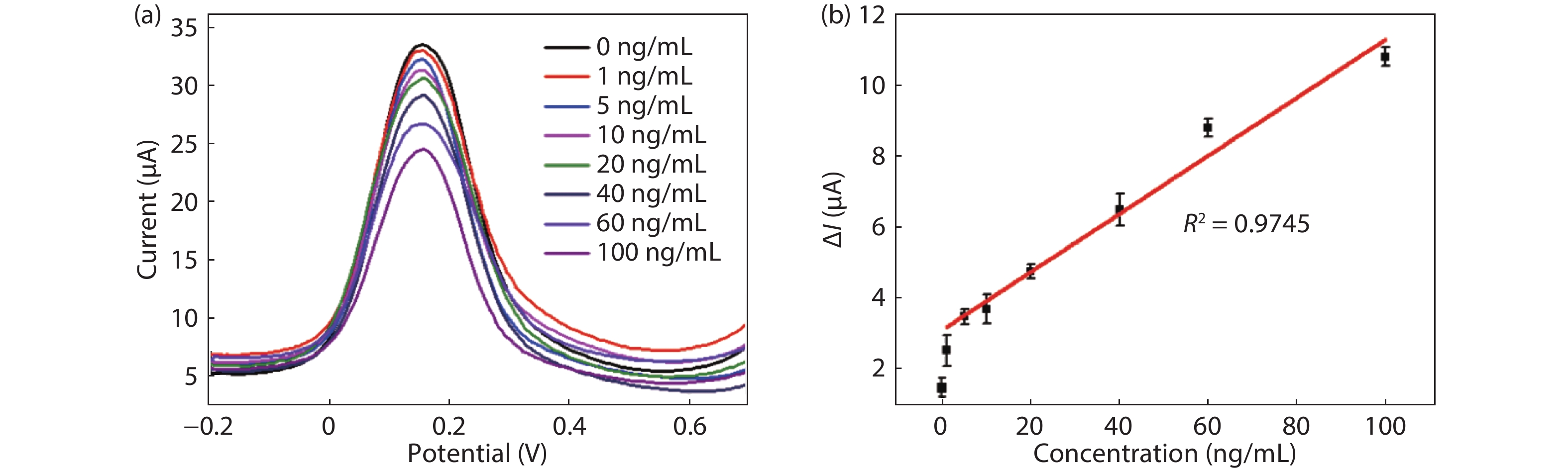
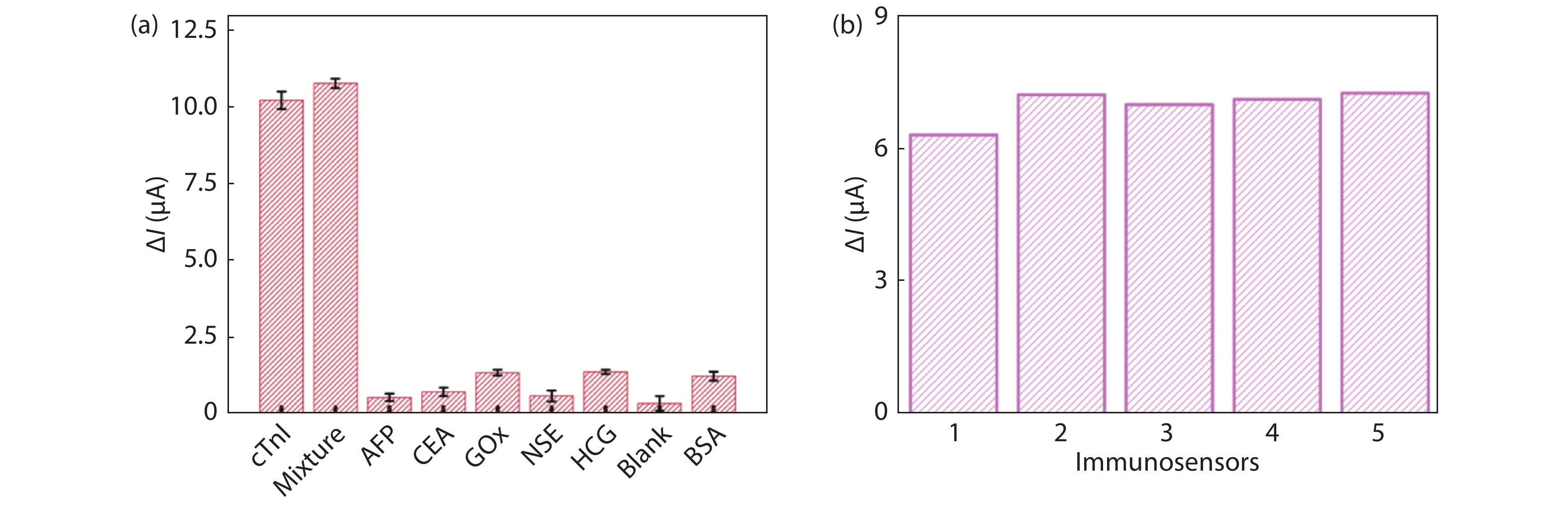
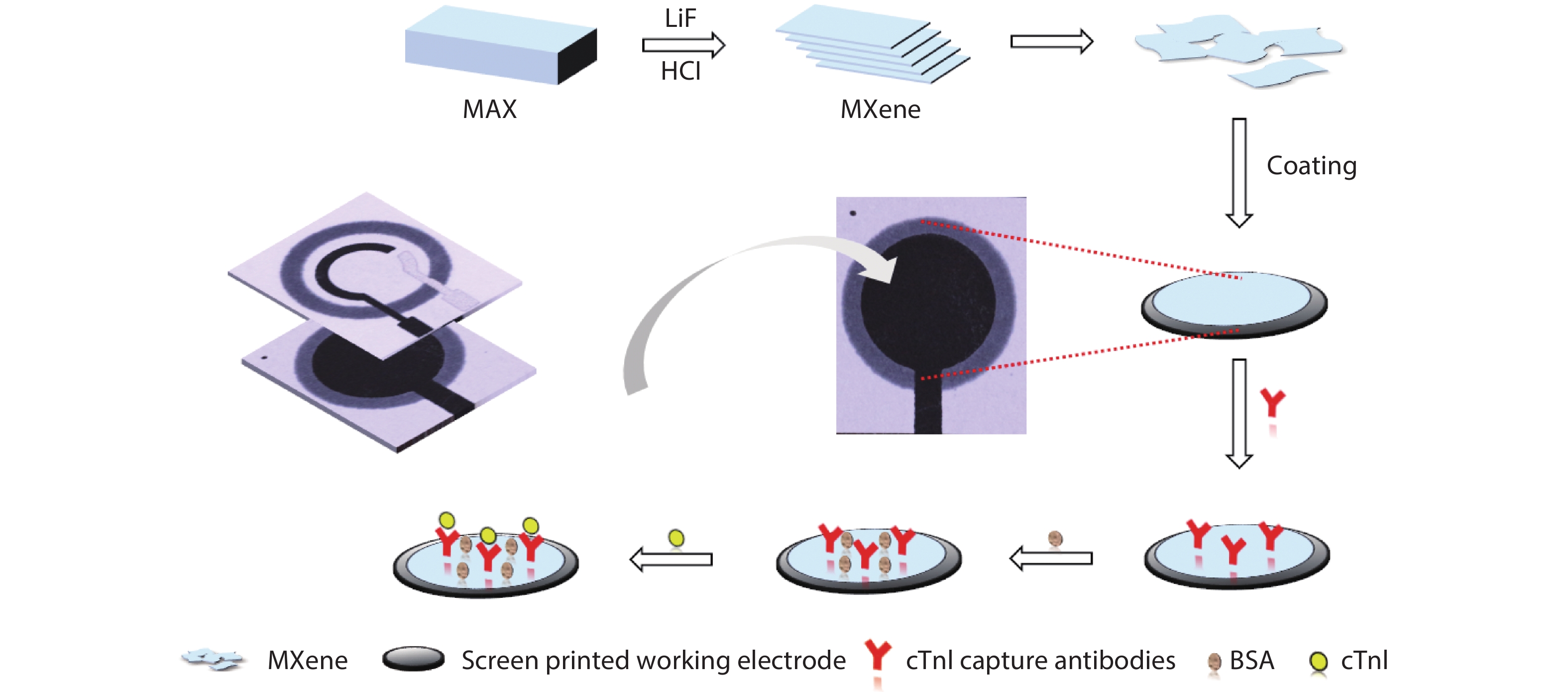
Convenient, rapid, and accurate detection of cardiac troponin I (cTnI) is crucial in early diagnosis of acute myocardial infarction (AMI). A paper-based electrochemical immunosensor is a promising choice in this field, because of the flexibility, porosity, and cost-efficacy of the paper. However, paper is poor in electronic conductivity and surface functionality. Herein, we report a paper-based electrochemical immunosensor for the label-free detection of cTnI with the working electrode modified by MXene (Ti3C2) nanosheets. In order to immobilize the bio-receptor (anti-cTnI) on the MXene-modified working electrode, the MXene nanosheets were functionalized by aminosilane, and the functionalized MXene was immobilized onto the surface of the working electrode through Nafion. The large surface area of the MXene nanosheets facilitates the immobilization of antibodies, and the excellent conductivity facilitates the electron transfer between the electrochemical species and the underlying electrode surface. As a result, the paper-based immunosensor could detect cTnI within a wide range of 5–100 ng/mL with a detection limit of 0.58 ng/mL. The immunosensor also shows outstanding selectivity and good repeatability. Our MXene-modified paper-based electrochemical immunosensor enables fast and sensitive detection of cTnI, which may be used in real-time and cost-efficient monitoring of AMI diseases in clinics. -
References
[1] Mohammed M I, Desmulliez M P Y. Lab-on-a-chip based immunosensor principles and technologies for the detection of cardiac biomarkers: a review. Lab Chip, 2011, 11(4), 569 doi: 10.1039/C0LC00204F[2] Ko S, Kim B, Jo S S, et al. Electrochemical detection of cardiac troponin I using a microchip with the surface-functionalized poly(dimethylsiloxane) channel. Biosens Bioelectron, 2007, 23(1), 51 doi: 10.1016/j.bios.2007.03.013[3] Guo X Y, Zong L J, Jiao Y C, et al. Signal-enhanced detection of multiplexed cardiac biomarkers by a paper-based fluorogenic immunodevice integrated with zinc oxide nanowires. Anal Chem, 2019, 91(14), 9300 doi: 10.1021/acs.analchem.9b02557[4] Zhang C, Du P F, Jiang Z J, et al. A simple and sensitive competitive bio-barcode immunoassay for triazophos based on multi-modified gold nanoparticles and fluorescent signal amplification. Anal Chim Acta, 2018, 999, 123 doi: 10.1016/j.aca.2017.10.032[5] Kim H J, Shelver W L, Hwang E C, et al. Automated flow fluorescent immunoassay for part per trillion detection of the neonicotinoid insecticide thiamethoxam. Anal Chim Acta, 2006, 571(1), 66 doi: 10.1016/j.aca.2006.04.084[6] Wang M H, Liu J J, Qin X L, et al. Electrochemiluminescence detection of cardiac troponin I based on Au-Ag alloy nanourchins. Analyst, 2020, 145(3), 873 doi: 10.1039/C9AN01904A[7] Yang Z J, Shen J, Li J, et al. An ultrasensitive streptavidin-functionalized carbon nanotubes platform for chemiluminescent immunoassay. Anal Chim Acta, 2013, 774, 85 doi: 10.1016/j.aca.2013.02.041[8] Zhao Y J, Liu X H, Li J, et al. Microfluidic chip-based silver nanoparticles aptasensor for colorimetric detection of thrombin. Talanta, 2016, 150, 81 doi: 10.1016/j.talanta.2015.09.013[9] Gao L, Yang Q F, Wu P. Recent advances in nanomaterial-enhanced enzyme-linked immunosorbent assays. Analyst, 2020, 145(12), 4069 doi: 10.1039/D0AN00597E[10] Martinez A W, Phillips S T, Butte M J, et al. Patterned paper as a platform for inexpensive, low-Volume, portable bioassays. Angew Chem Int Ed, 2007, 46(8), 1318 doi: 10.1002/anie.200603817[11] Jiao Y C, Du C, Zong L J, et al. 3D vertical-flow paper-based device for simultaneous detection of multiple cancer biomarkers by fluorescent immunoassay. Sens Actuators B, 2020, 306, 127239 doi: 10.1016/j.snb.2019.127239[12] Nair R R. Organic electrochemical transistor on paper for the detection of halide anions in biological analytes. Flex Print Electron, 2020, 5(4), 045004 doi: 10.1088/2058-8585/abc9c9[13] Yuan W, Wu X Z, Gu W B, et al. Printed stretchable circuit on soft elastic substrate for wearable application. J Semicond, 2018, 39(1), 015002 doi: 10.1088/1674-4926/39/1/015002[14] Zong L J, Jiao Y C, Guo X Y, et al. Paper-based fluorescent immunoassay for highly sensitive and selective detection of norfloxacin in milk at picogram level. Talanta, 2019, 195, 333 doi: 10.1016/j.talanta.2018.11.073[15] Zong L J, Han Y F, Gao L, et al. A transparent paper-based platform for multiplexed bioassays by wavelength-dependent absorbance/transmittance. Analyst, 2019, 144(24), 7157 doi: 10.1039/C9AN01647C[16] Wang D R, Mei Y F, Huang G S. Printable inorganic nanomaterials for flexible transparent electrodes: from synthesis to application. J Semicond, 2018, 39(1), 011002 doi: 10.1088/1674-4926/39/1/011002[17] Li Z Y, Huang X, Lu G. Recent developments of flexible and transparent SERS substrates. J Mater Chem C, 2020, 8(12), 3956 doi: 10.1039/D0TC00002G[18] Zou M Z, Ma Y, Yuan X, et al. Flexible devices: from materials, architectures to applications. J Semicond, 2018, 39(1), 011010 doi: 10.1088/1674-4926/39/1/011010[19] Wang H, Li H, Huang Y, et al. A label-free electrochemical biosensor for highly sensitive detection of gliotoxin based on DNA nanostructure/MXene nanocomplexes. Biosens Bioelectron, 2019, 142, 111531 doi: 10.1016/j.bios.2019.111531[20] Naguib M, Kurtoglu M, Presser V, et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater, 2011, 23(37), 4248 doi: 10.1002/adma.201102306[21] Ren Z H, Qi D C, Sonar P, et al. Flexible sensors based on hybrid materials. J Semicond, 2020, 41(4), 040402 doi: 10.1088/1674-4926/41/4/040402[22] Naguib M, Mochalin V N, Barsoum M W, et al. 25th anniversary article: MXenes: a new family of two-dimensional materials. Adv Mater, 2014, 26(7), 992 doi: 10.1002/adma.201304138[23] Naguib M, Mashtalir O, Carle J, et al. Two-dimensional transition metal carbides. ACS Nano, 2012, 6(2), 1322 doi: 10.1021/nn204153h[24] Neupane G P, Yildirim T, Zhang L L, et al. Emerging 2D MXene/organic heterostructures for future nanodevices. Adv Funct Mater, 2020, 30(52), 2005238 doi: 10.1002/adfm.202005238[25] Xiao R, Zhao C X, Zou Z Y, et al. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl Catal B, 2020, 268, 118382 doi: 10.1016/j.apcatb.2019.118382[26] Meng Y, Ho J C. MXene-based wearable biosensor. J Semicond, 2019, 40(11), 110202 doi: 10.1088/1674-4926/40/11/110202[27] Li Z, Wu Y. 2D early transition metal carbides (MXenes) for catalysis. Small, 2019, 15(29), 1804736 doi: 10.1002/smll.201804736[28] Ahmed B, EI Ghazaly A, Rosen J. i-MXenes for energy storage and catalysis. Adv Funct Mater, 2020, 30(47), 2000894 doi: 10.1002/adfm.202000894[29] Huang K, Li Z J, Lin J, et al. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem Soc Rev, 2018, 47(14), 5109 doi: 10.1039/C7CS00838D[30] Zhang H X, Wang Z H, Zhang Q X, et al. Ti3C2 MXenes nanosheets catalyzed highly efficient electrogenerated chemiluminescence biosensor for the detection of exosomes. Biosens Bioelectron, 2019, 124, 184 doi: 10.1016/j.bios.2018.10.016[31] Jiang Q, Kurra N, Alhabeb M, et al. All pseudocapacitive MXene-RuO2 asymmetric supercapacitors. Adv Energy Mater, 2018, 8(13), 1703043 doi: 10.1002/aenm.201703043[32] Yang Q Y, Xu Z, Fang B, et al. MXene/graphene hybrid fibers for high performance flexible supercapacitors. J Mater Chem A, 2017, 5(42), 22113 doi: 10.1039/C7TA07999K[33] Du Y T, Kan X, Yang F, et al. MXene/graphene heterostructures as high-performance electrodes for Li-ion batteries. ACS Appl Mater Inter, 2018, 10(38), 32867 doi: 10.1021/acsami.8b10729[34] Ahmed B, Anjum D H, Gogotsi Y, et al. Atomic layer deposition of SnO2 on MXene for Li-ion battery anodes. Nano Energy, 2017, 34, 249 doi: 10.1016/j.nanoen.2017.02.043[35] Ahmed B, Anjum D H, Hedhili M N, et al. H2O2 assisted room temperature oxidation of Ti2C MXene for Li-ion battery anodes. Nanoscale, 2016, 8(14), 7580 doi: 10.1039/C6NR00002A[36] Wang Y, Luo J P, Liu J T, et al. Electrochemical integrated paper-based immunosensor modified with multi-walled carbon nanotubes nanocomposites for point-of-care testing, of 17 beta-estradiol. Biosens Bioelectron, 2018, 107, 47 doi: 10.1016/j.bios.2018.02.012[37] Zhang G L, Wang T C, Xu Z H, et al. Synthesis of amino-functionalized Ti3C2Tx MXene by alkalization-grafting modification for efficient lead adsorption. Chem Commun, 2020, 56, 11283 doi: 10.1039/D0CC04265J[38] White L D, Tripp C P. Reaction of (3-Aminopropyl)dimethylethoxysilane with amine catalysts on silica surfaces. J Colloid Interf Sci, 2000, 232, 400 doi: 10.1006/jcis.2000.7224[39] Lei J M, Chen X M. RuO2/MnO2 composite materials for high-performance supercapacitor electrodes. J Semicond, 2015, 36(8), 083006 doi: 10.1088/1674-4926/36/8/083006[40] Wang S P, Wang J J, Zhu Y F, et al. Cantilever with immobilized antibody for liver cancer biomarker detection. J Semicond, 2014, 35(10), 104008 doi: 10.1088/1674-4926/35/10/104008[41] Miao L Y, Jiao L, Tang Q R, et al. A nanozyme-linked immunosorbent assay for dual-modal colorimetric and ratiometric fluorescent detection of cardiac troponin I. Sens Actuators B, 2019, 288, 60 doi: 10.1016/j.snb.2019.02.111[42] Lee S, Kang S H. Quenching effect on gold nano-patterned cardiac troponin I chip by total internal reflection fluorescencemicroscopy. Talanta, 2013, 104, 32 doi: 10.1016/j.talanta.2012.11.014[43] Torabi F, Mobini F H R, Danielsson B, et al. Development of a plasma panel test for detection of human myocardial. Biosens Bioelectron, 2007, 22, 1218 doi: 10.1016/j.bios.2006.04.030[44] Gong M M, Sinton D. Turning the page: advancing paper-based microfluidics for broad diagnostic application. Chem Rev, 2017, 117, 8447 doi: 10.1021/acs.chemrev.7b00024[45] Noviana E, McCord C P, Clark K M, et al. Electrochemical paper-based devices: sensing approaches and progress toward practical applications. Lab Chip, 2019, 20, 9 doi: 10.1039/C9LC00903E -
Supplements
 21020007suppl.pdf
21020007suppl.pdf

-
Proportional views





 DownLoad:
DownLoad: