| Citation: |
Agbolade Lukman Olatomiwa, Tijjani Adam, Subash C. B. Gopinath, Sanusi Yekinni Kolawole, Oyeshola Hakeem Olayinka, U. Hashim. Graphene synthesis, fabrication, characterization based on bottom-up and top-down approaches: An overview[J]. Journal of Semiconductors, 2022, 43(6): 061101. doi: 10.1088/1674-4926/43/6/061101
****
A L Olatomiwa, T Adam, S C B Gopinath, S Y Kolawole, O H Olayinka, U Hashim. Graphene synthesis, fabrication, characterization based on bottom-up and top-down approaches: An overview[J]. J. Semicond, 2022, 43(6): 061101. doi: 10.1088/1674-4926/43/6/061101
|
Graphene synthesis, fabrication, characterization based on bottom-up and top-down approaches: An overview
DOI: 10.1088/1674-4926/43/6/061101
More Information
-
Abstract
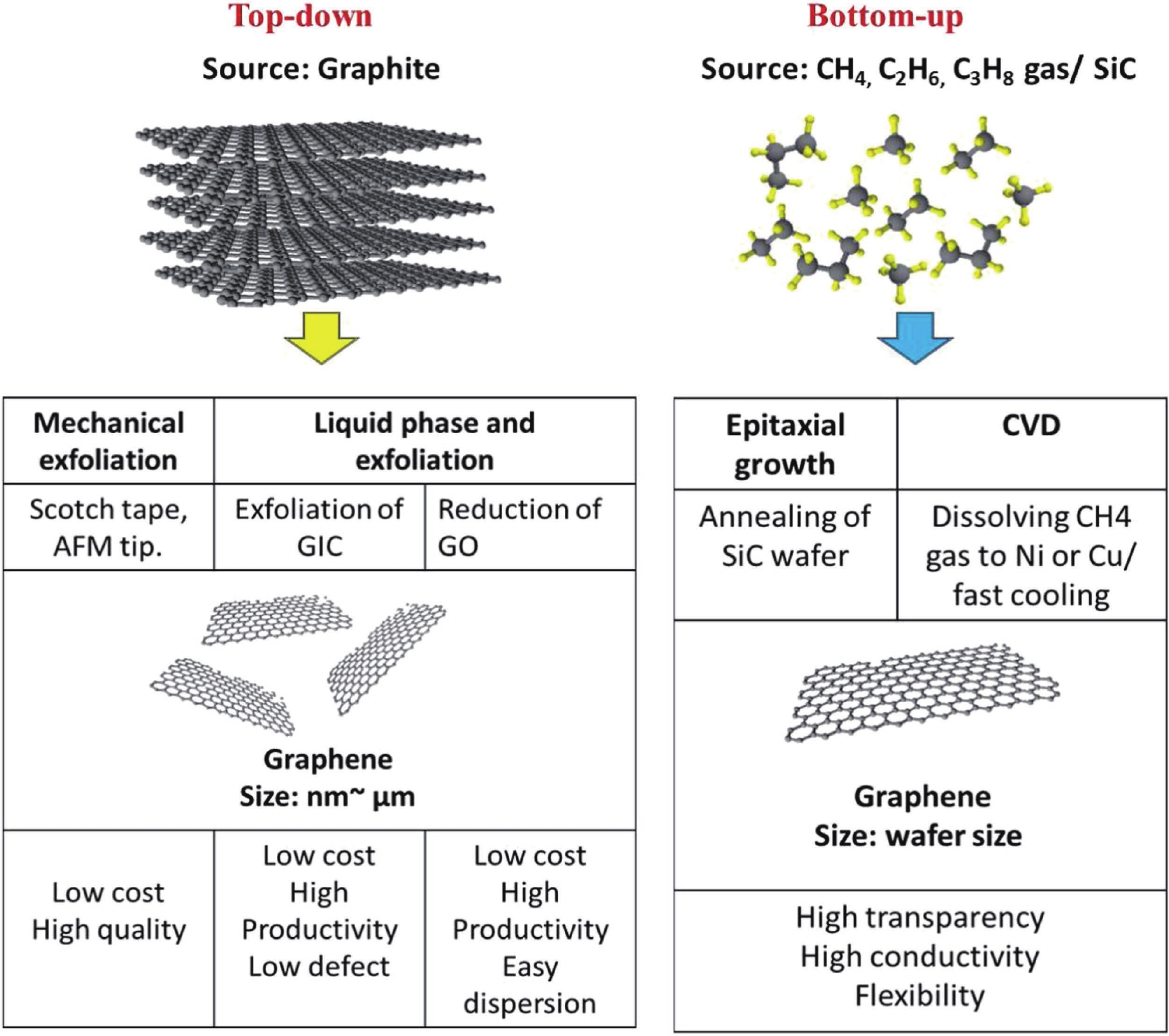
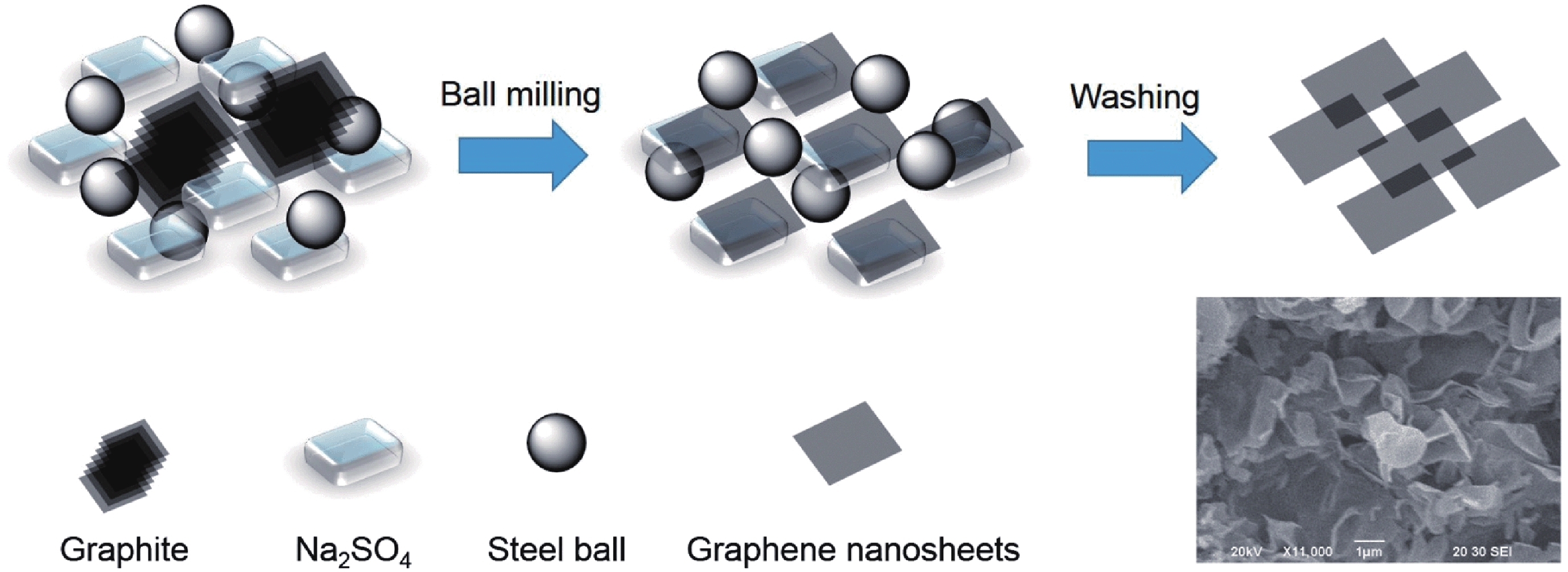
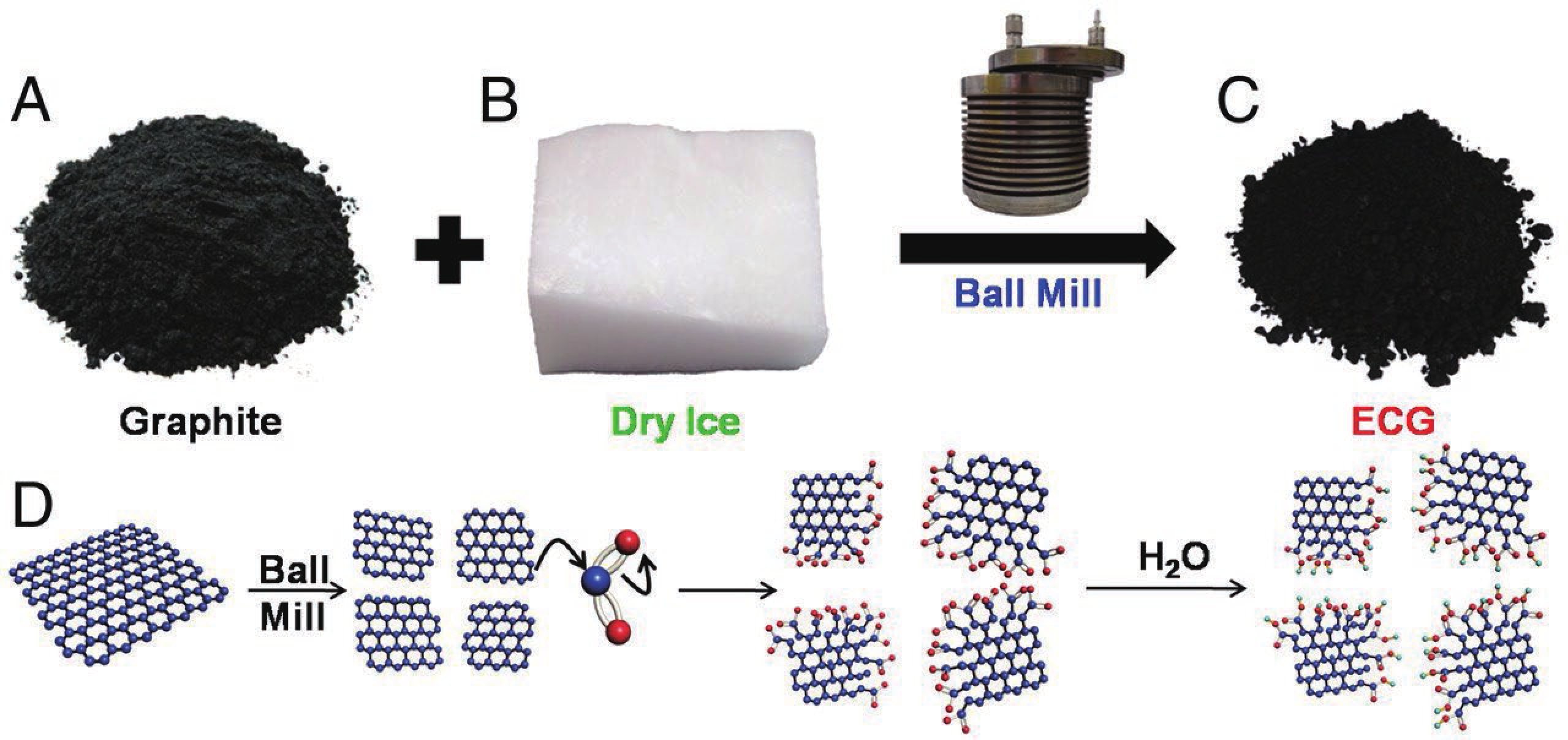
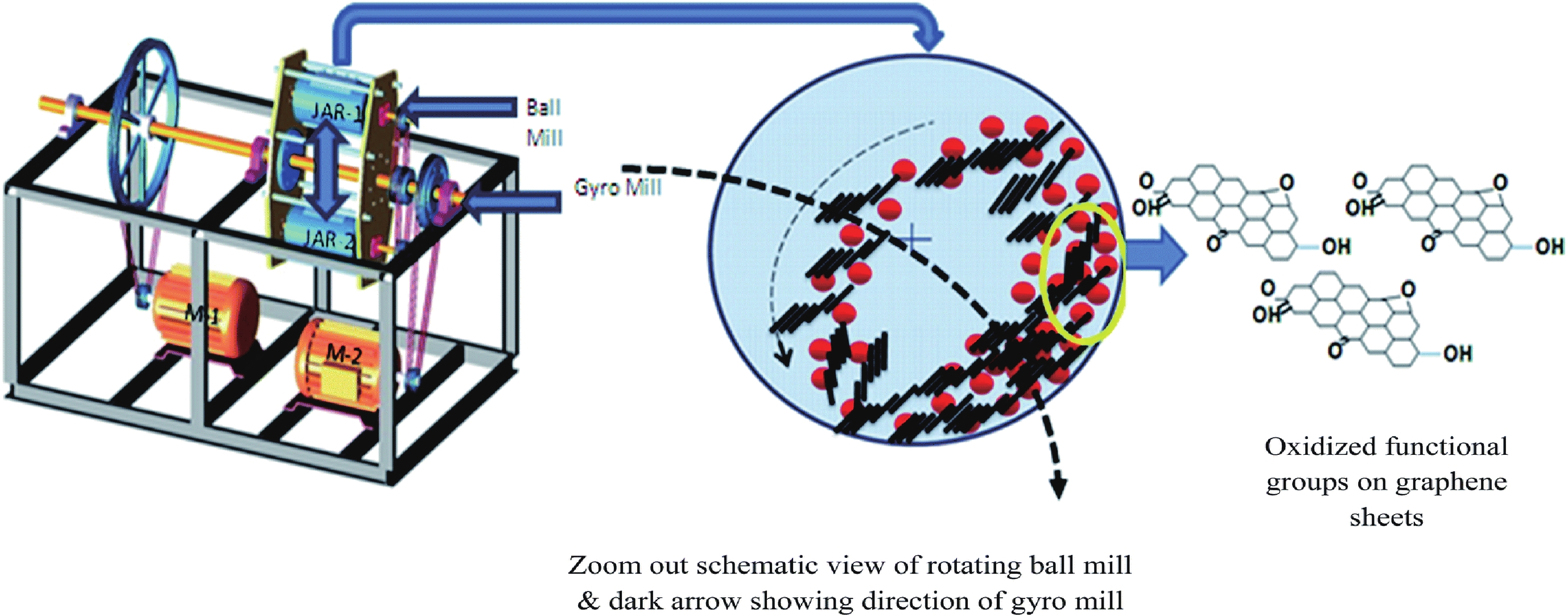
This study presents an overview on graphene synthesis, fabrication and different characterization techniques utilized in the production. Since its discovery in 2004 by Andre Geim and Kostya Novoselov several research articles have been published globally to this effect, owing to graphene’s extraordinary, and exclusive characteristics which include optical transparency, excellent thermal, and mechanical properties. The properties and applications of this two-dimensional carbon crystal composed of single-layered material have created new avenues for the development of high-performance future electronics and technologies in energy storage and conversion for the sustainable energy. However, despite its potential and current status globally the difficulty in the production of monolayer graphene sheet still persists. Therefore, this review highlighted two approaches in the synthesis of graphene, which are the top-down and bottom-up approaches and examined the advantages and failings of the methods involved. In addition, the prospects and failings of these methods are investigated, as they are essential in optimizing the production method of graphene vital for expanding the yield, and producing high-quality graphene.-
Keywords:
- two-dimensional material,
- nanomaterial,
- carbon material,
- nanostructure
-
References
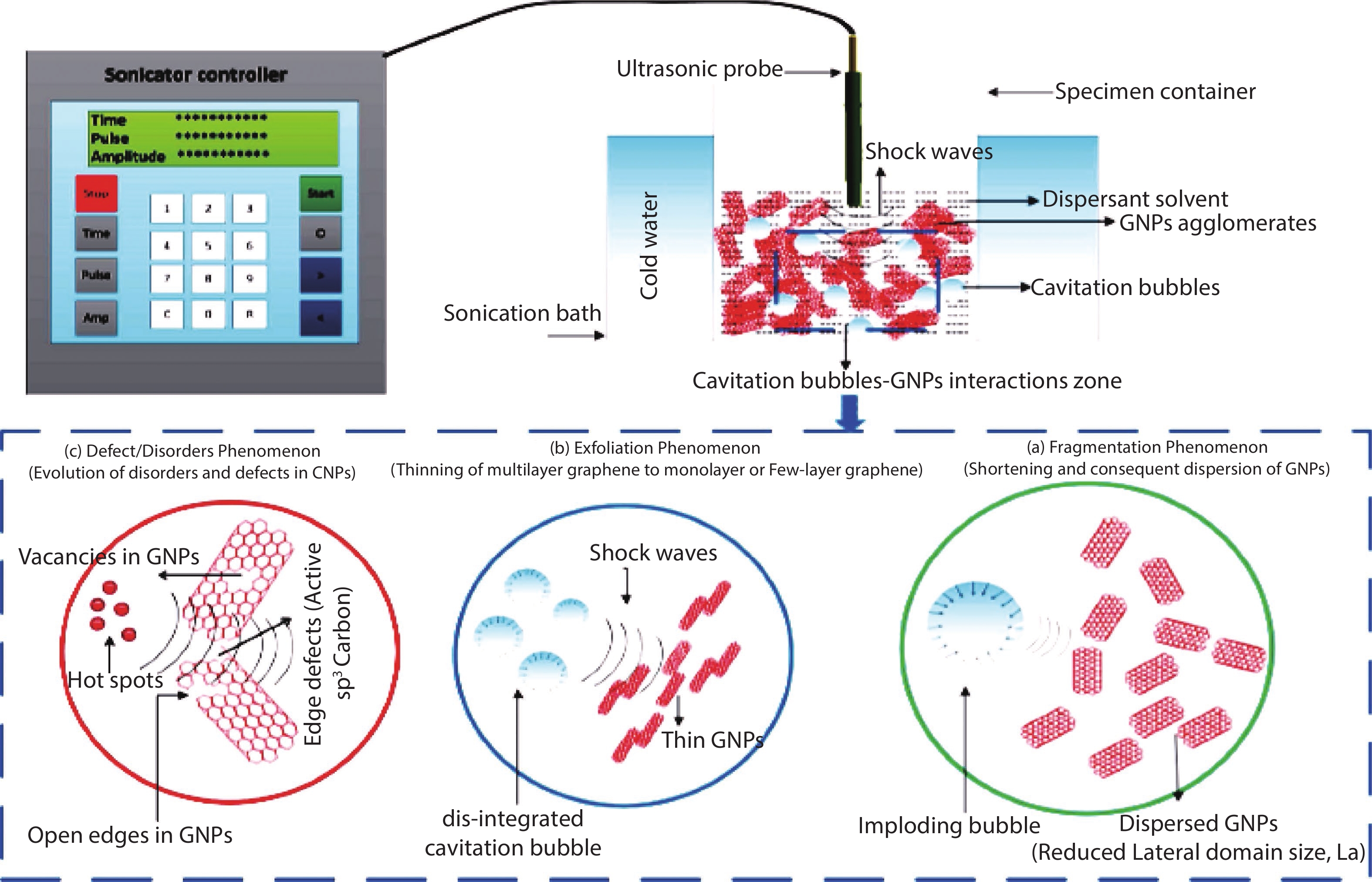
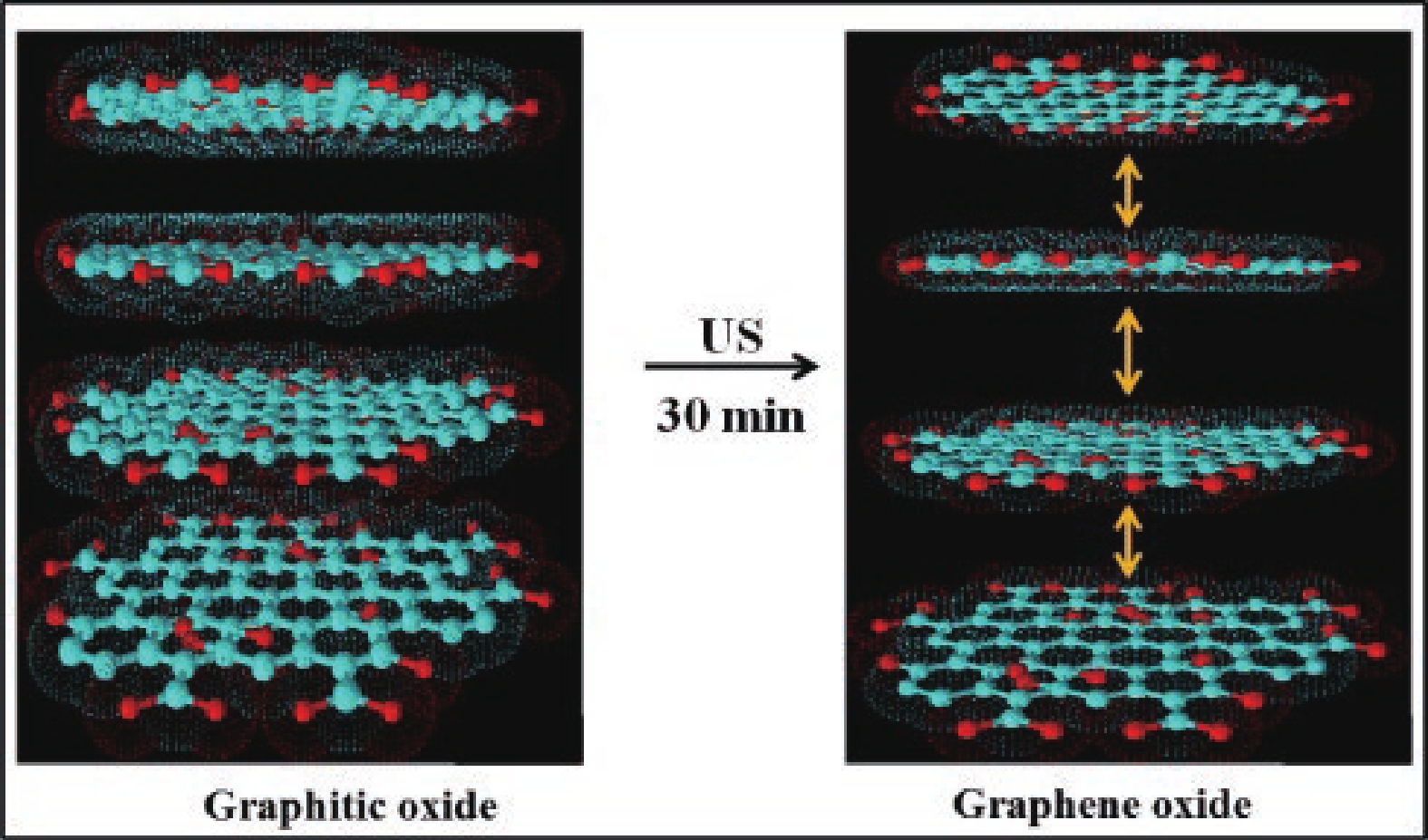
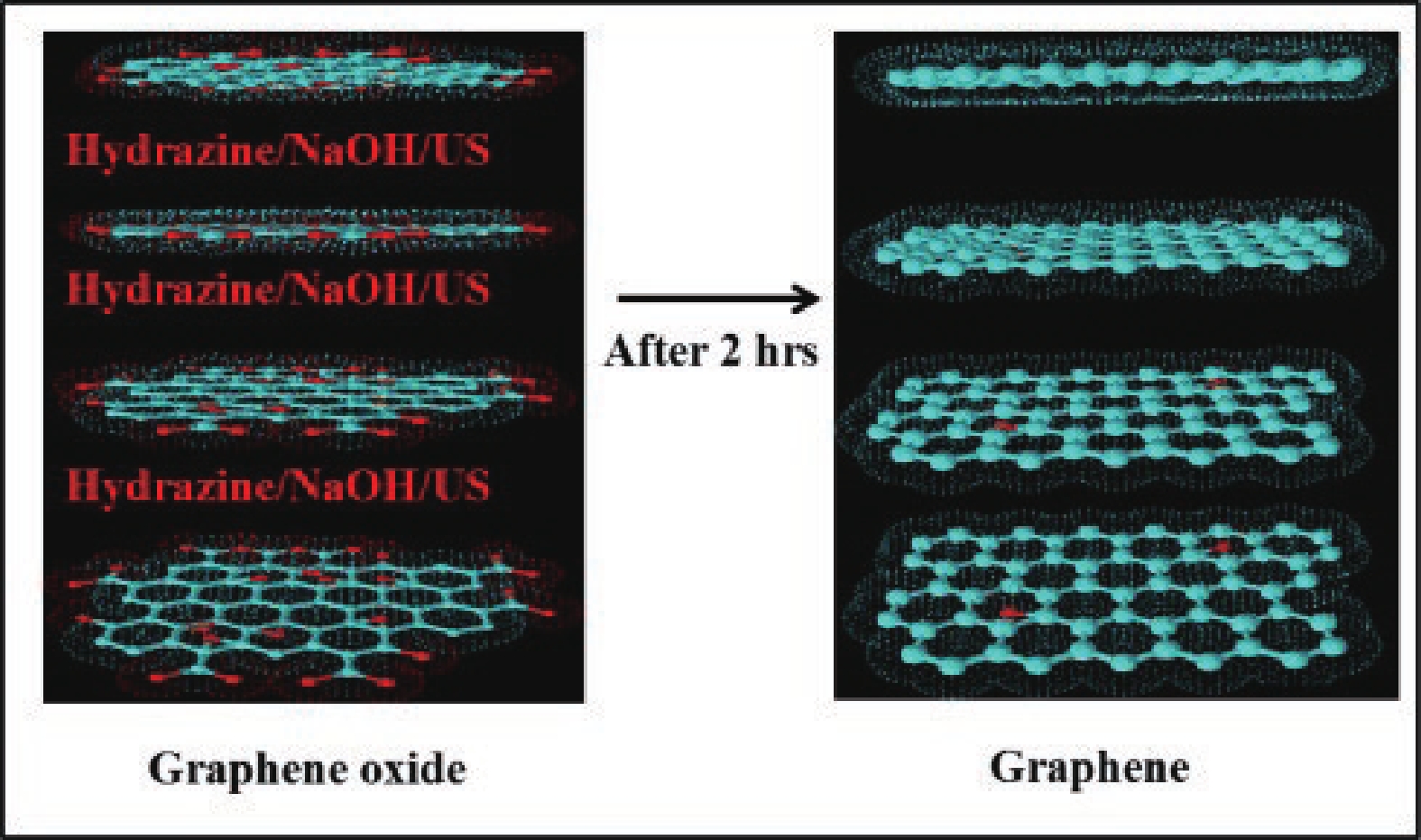
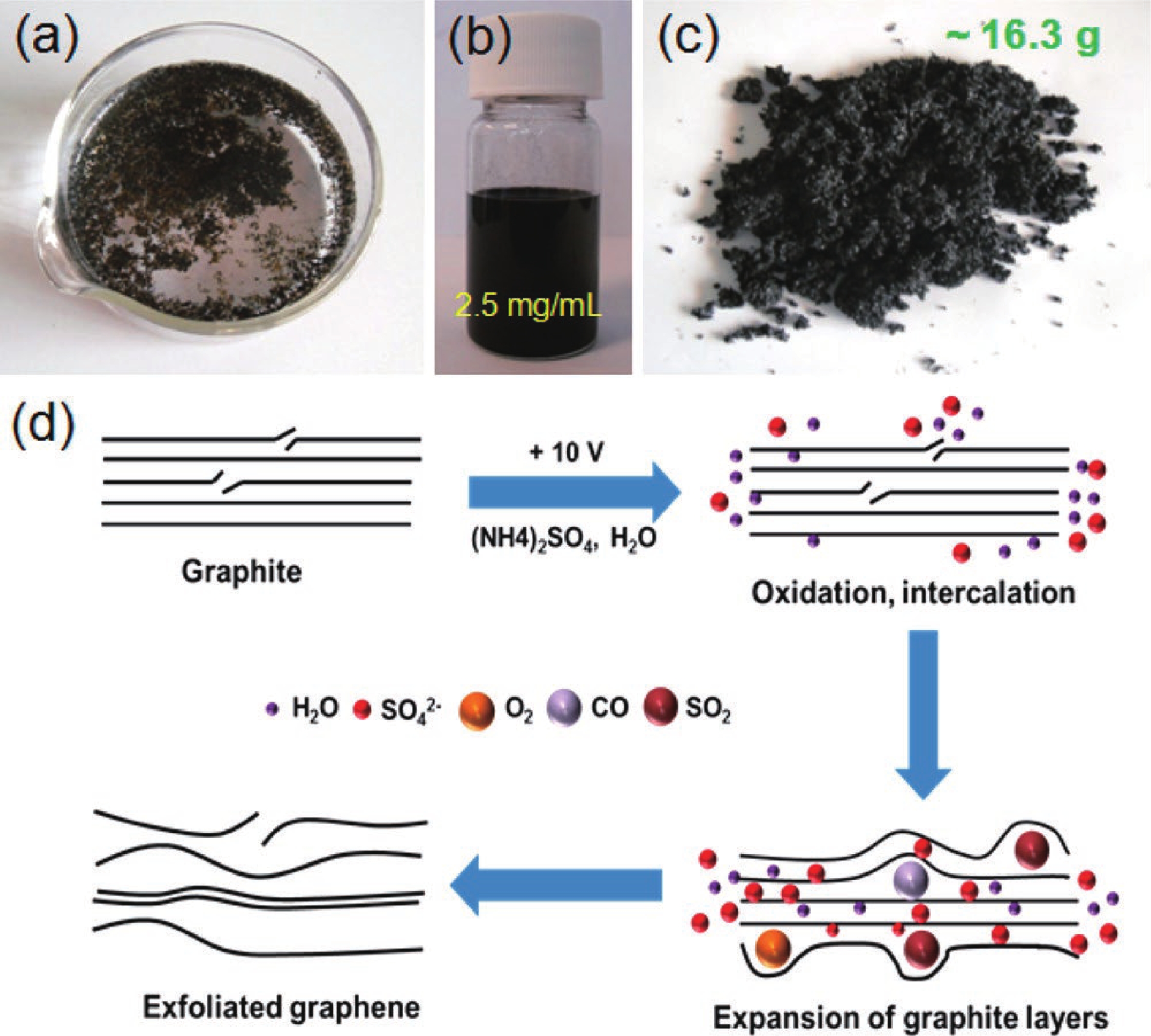
[1] Katsnelson M I. Graphene. Encyclopedia Britannica, 2016. https://www.britannica.com/science/graphene[2] Liu M. Controlled synthesis and scanning tunneling microscopy study of graphene-based heterostructures. Springer, 2017[3] Chabot V, Higgins D, Yu A P, et al. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ Sci, 2014, 7, 1564 doi: 10.1039/c3ee43385d[4] Novoselov K S, Fal'ko V I, Colombo L, et al. A roadmap for graphene. Nature, 2012, 490, 192 doi: 10.1038/nature11458[5] Balandin A A. Thermal properties of graphene and nanostructured carbon materials. Nat Mater, 2011, 10, 569 doi: 10.1038/nmat3064[6] Cao X, Shi J J, Zhang M, et al. Band gap opening of graphene by forming heterojunctions with the 2D carbonitrides nitrogenated holey graphene, g-C3N4, and g-CN: Electric field effect. J Phys Chem C, 2016, 120, 11299 doi: 10.1021/acs.jpcc.6b03308[7] Sahu S, Rout G C. Band gap opening in graphene: A short theoretical study. Int Nano Lett, 2017, 7, 81 doi: 10.1007/s40089-017-0203-5[8] Li X S, Colombo L, Ruoff R S. Synthesis of graphene films on copper foils by chemical vapor deposition. Adv Mater, 2016, 28, 6247 doi: 10.1002/adma.201504760[9] Stankovich S, Dikin D A, Piner R D, et al. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon, 2007, 45, 1558 doi: 10.1016/j.carbon.2007.02.034[10] Berger C, Song Z M, Li T B, et al. Ultrathin epitaxial graphite: 2D electron gas properties and a route toward graphene-based nanoelectronics. J Phys Chem B, 2004, 108, 19912 doi: 10.1021/jp040650f[11] Bhuyan M S A, Uddin M N, Islam M M, et al. Synthesis of graphene. Int Nano Lett, 2016, 6, 65 doi: 10.1007/s40089-015-0176-1[12] Kar S, Saha S, Dutta S, et al. A comprehensive review over green synthesis of graphene. Int J Res Sci Innov, 2018, 5, 2321[13] Fernández-Merino M J, Guardia L, Paredes J I, et al. Vitamin C is an ideal substitute for hydrazine in the reduction of graphene oxide suspensions. J Phys Chem C, 2010, 114, 6426 doi: 10.1021/jp100603h[14] Kartick B, Srivastava S K, Srivastava I. Green synthesis of graphene. J Nanosci Nanotechnol, 2013, 13, 4320 doi: 10.1166/jnn.2013.7461[15] Choi W, Lahiri I, Seelaboyina R, et al. Synthesis of graphene and its applications: A review. Crit Rev Solid State Mater Sci, 2010, 35, 52 doi: 10.1080/10408430903505036[16] Eizenberg M, Blakely J M. Carbon monolayer phase condensation on Ni(111). Surf Sci, 1979, 82, 228 doi: 10.1016/0039-6028(79)90330-3[17] Oshima C, Nagashima A. Ultra-thin epitaxial films of graphite and hexagonal boron nitride on solid surfaces. J Phys: Condens Matter, 1997, 9, 1 doi: 10.1088/0953-8984/9/1/004[18] Novoselov K S, Jiang D, Schedin F, et al. Two-dimensional atomic crystals. PNAS, 2005, 102, 10451 doi: 10.1073/pnas.0502848102[19] Rokuta E, Hasegawa Y, Itoh A, et al. Vibrational spectra of the monolayer films of hexagonal boron nitride and graphite on faceted Ni(755). Surf Sci, 1999, 427/428, 97 doi: 10.1016/S0039-6028(99)00241-1[20] Liu S D, Hui K S, Hui K N. Review of the green synthesis of metal/graphene composites for energy conversion, sensor, environmental, and bioelectronic applications. In: Advanced Bioelectronic Materials. John Wiley & Sons, Inc., 2015, 427[21] Li X, Cai W, An J, et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science, 2009, 324, 1312 doi: 10.1126/science.1171245[22] Das V K, Shifrina Z B, Bronstein L M. Graphene and graphene-like materials in biomass conversion: Paving the way to the future. J Mater Chem A, 2017, 5, 25131 doi: 10.1039/C7TA09418C[23] Mahmoudi T, Wang Y S, Hahn Y B. Graphene and its derivatives for solar cells application. Nano Energy, 2018, 47, 51 doi: 10.1016/j.nanoen.2018.02.047[24] Singh J, Dutta T, Kim K H, et al. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J Nanobiotechnol, 2018, 16, 84 doi: 10.1186/s12951-018-0408-4[25] Ghatule M P, Devare U A. Study and analysis on synthesis of graphene and its applications in solar cells. Int J Innov Res Adv Eng, 2016, 1, 184[26] Zhang Z S, Fraser A, Ye S Y, et al. Top-down bottom-up graphene synthesis. Nano Futur, 2019, 3, 042003 doi: 10.1088/2399-1984/ab4eff[27] Walimbe P, Chaudhari M. State-of-the-art advancements in studies and applications of graphene: A comprehensive review. Mater Today Sustain, 2019, 6, 100026 doi: 10.1016/j.mtsust.2019.100026[28] Bunch J S, Verbridge S S, Alden J S, et al. Impermeable atomic membranes from graphene sheets. Nano Lett, 2008, 8, 2458 doi: 10.1021/nl801457b[29] Rozpłoch F, Patyk J, Stankowski J. Graphenes bonding forces in graphite. Acta Phys Pol A, 2007, 112, 557 doi: 10.12693/APhysPolA.112.557[30] Yin L, Deng C, Deng F, et al. Analysis of the interaction energies between and within graphite particles during mechanical exfoliation. New Carbon Mater, 2018, 33, 449 doi: 10.1016/S1872-5805(18)60351-8[31] Du W C, Geng H B, Yang Y, et al. Pristine graphene for advanced electrochemical energy applications. J Power Sources, 2019, 437, 226899 doi: 10.1016/j.jpowsour.2019.226899[32] Cai C J, Sang N N, Shen Z G, et al. Facile and size-controllable preparation of graphene oxide nanosheets using high shear method and ultrasonic method. J Exp Nanosci, 2017, 12, 247 doi: 10.1080/17458080.2017.1303853[33] El-Eskandarany M S. The history and necessity of mechanical alloying. In: Mechanical Alloying. Elsevier, 2017[34] Beladi-Mousavi S M, Sadaf S, Walder L, et al. Poly(vinylferrocene)-reduced graphene oxide as a high power/high capacity cathodic battery material. Adv Energy Mater, 2016, 6, 1600108 doi: 10.1002/aenm.201600108[35] Hassanzadeh N, Sadrnezhaad S K, Chen G H. Ball mill assisted synthesis of Na3MnCO3PO4 nanoparticles anchored on reduced graphene oxide for sodium ion battery cathodes. Electrochim Acta, 2016, 220, 683 doi: 10.1016/j.electacta.2016.10.160[36] Lv Y Y, Yu L S, Jiang C M, et al. Synthesis of graphene nanosheet powder with layer number control via a soluble salt-assisted route. RSC Adv, 2014, 4, 13350 doi: 10.1039/c3ra45060k[37] Kairi M I, Dayou S, Kairi N I, et al. Toward high production of graphene flakes–a review on recent developments in their synthesis methods and scalability. J Mater Chem A, 2018, 6, 15010 doi: 10.1039/C8TA04255A[38] Jeon I Y, Shin Y R, Sohn G J, et al. Edge-carboxylated graphene nanosheets via ball milling. PNAS, 2012, 109, 5588 doi: 10.1073/pnas.1116897109[39] Zhao W F, Fang M, Wu F R, et al. Preparation of graphene by exfoliation of graphite using wet ball milling. J Mater Chem, 2010, 20, 5817 doi: 10.1039/c0jm01354d[40] Dash P, Dash T, Rout T K, et al. Preparation of graphene oxide by dry planetary ball milling process from natural graphite. RSC Adv, 2016, 6, 12657 doi: 10.1039/C5RA26491J[41] Casallas Caicedo F M, Vera López E, Agarwal A, et al. Synthesis of graphene oxide from graphite by ball milling. Diam Relat Mater, 2020, 109, 108064 doi: 10.1016/j.diamond.2020.108064[42] Xu Y Y, Cao H Z, Xue Y Q, et al. Liquid-phase exfoliation of graphene: An overview on exfoliation media, techniques, and challenges. Nanomaterials, 2018, 8, 942 doi: 10.3390/nano8110942[43] Ciesielski A, Samorì P. Graphene via sonication assisted liquid-phase exfoliation. Chem Soc Rev, 2014, 43, 381 doi: 10.1039/C3CS60217F[44] Cai X, Jiang Z, Zhang X, et al. Effects of tip sonication parameters on liquid phase exfoliation of graphite into graphene nanoplatelets. Nanoscale Res Lett, 2018, 13, 241 doi: 10.1186/s11671-018-2648-5[45] Savun-Hekimoğlu B. A review on sonochemistry and its environmental applications. Acoustics, 2020, 2, 766 doi: 10.3390/acoustics2040042[46] Baig Z, Mamat O, Mustapha M, et al. Investigation of tip sonication effects on structural quality of graphene nanoplatelets (GNPs) for superior solvent dispersion. Ultrason Sonochem, 2018, 45, 133 doi: 10.1016/j.ultsonch.2018.03.007[47] May P, Khan U, O'Neill A, et al. Approaching the theoretical limit for reinforcing polymers with graphene. J Mater Chem, 2012, 22, 1278 doi: 10.1039/C1JM15467B[48] Krishnamoorthy K, Kim G S, Kim S J. Graphene nanosheets: Ultrasound assisted synthesis and characterization. Ultrason Sonochem, 2013, 20, 644 doi: 10.1016/j.ultsonch.2012.09.007[49] Gürünlü B, Taşdelen-Yücedağ Ç, Bayramoğlu M. Graphene synthesis by ultrasound energy-assisted exfoliation of graphite in various solvents. Crystals, 2020, 10, 1037 doi: 10.3390/cryst10111037[50] Yu P, Lowe S E, Simon G P, et al. Electrochemical exfoliation of graphite and production of functional graphene. Curr Opin Colloid Interface Sci, 2015, 20, 329 doi: 10.1016/j.cocis.2015.10.007[51] Li L, Zhang D, Deng J P, et al. Review—preparation and application of graphene-based hybrid materials through electrochemical exfoliation. J Electrochem Soc, 2020, 167, 086511 doi: 10.1149/1945-7111/ab933b[52] Achee T C, Sun W M, Hope J T, et al. High-yield scalable graphene nanosheet production from compressed graphite using electrochemical exfoliation. Sci Rep, 2018, 8, 14525 doi: 10.1038/s41598-018-32741-3[53] Parvez K, Wu Z S, Li R J, et al. Exfoliation of graphite into graphene in aqueous solutions of inorganic salts. J Am Chem Soc, 2014, 136, 6083 doi: 10.1021/ja5017156[54] Munuera J M, Paredes J I, Villar-Rodil S, et al. High quality, low-oxidized graphene via anodic exfoliation with table salt as an efficient oxidation-preventing co-electrolyte for water/oil remediation and capacitive energy storage applications. Appl Mater Today, 2018, 11, 246 doi: 10.1016/j.apmt.2018.03.002[55] Yang S, Lohe M R, Müllen K, et al. New-generation graphene from electrochemical approaches: Production and applications. Adv Mater, 2016, 28, 6213 doi: 10.1002/adma.201505326[56] Yang Y C, Lu F, Zhou Z, et al. Electrochemically cathodic exfoliation of graphene sheets in room temperature ionic liquids N-butyl, methylpyrrolidinium bis(trifluoromethylsulfonyl)imide and their electrochemical properties. Electrochim Acta, 2013, 113, 9 doi: 10.1016/j.electacta.2013.09.031[57] Dalal M H, Lee C Y, Wallace G G. Cathodic exfoliation of graphite into graphene nanoplatelets in aqueous solution of alkali metal salts. J Mater Sci, 2021, 56, 3612 doi: 10.1007/s10853-020-05468-8[58] Li L, Wang M Q, Guo J, et al. Regulation of radicals from electrochemical exfoliation of a double-graphite electrode to fabricate high-quality graphene. J Mater Chem C, 2018, 6, 6257 doi: 10.1039/C8TC01565A[59] Kouloumpis A, Spyrou K, Dimos K, et al. A bottom-up approach for the synthesis of highly ordered fullerene-intercalated graphene hybrids. Front Mater, 2015, 2, 10 doi: 10.3389/fmats.2015.00010[60] Price R J, Ladislaus P I, Smith G C, et al. A novel ‘bottom-up’synthesis of few-and multi-layer graphene platelets with partial oxidation via cavitation. Ultrasons Sonochem, 2019, 56, 466 doi: 10.1016/j.ultsonch.2019.03.020[61] Gürünlü B, Taşdelen Yücedağ Ç, Bayramoğlu M R. Green synthesis of graphene from graphite in molten salt medium. J Nanomater, 2020, 2020, 7029601 doi: 10.1155/2020/7029601[62] Tour J M. Top-down versus bottom-up fabrication of graphene-based electronics. Chem Mater, 2014, 26, 163 doi: 10.1021/cm402179h[63] Gupta B, Notarianni M, Mishra N, et al. Evolution of epitaxial graphene layers on 3C SiC/Si (1 1 1) as a function of annealing temperature in UHV. Carbon, 2014, 68, 563 doi: 10.1016/j.carbon.2013.11.035[64] Yu X Z, Hwang C G, Jozwiak C M, et al. New synthesis method for the growth of epitaxial graphene. J Electron Spectrosc Relat Phenom, 2011, 184, 100 doi: 10.1016/j.elspec.2010.12.034[65] Badami D V. Graphitization of α-silicon carbide. Nature, 1962, 193, 569 doi: 10.1038/193569a0[66] van Bommel A J, Crombeen J E, Tooren A V. LEED and Auger electron observations of the SiC(0001) surface. Surf Sci, 1975, 48, 463 doi: 10.1016/0039-6028(75)90419-7[67] de Heer W A, Berger C, Wu X S, et al. Epitaxial graphene. Solid State Commun, 2007, 143, 92 doi: 10.1016/j.ssc.2007.04.023[68] de Heer W A, Berger C, Ruan M, et al. Large area and structured epitaxial graphene produced by confinement controlled sublimation of silicon carbide. PNAS, 2011, 108, 16900 doi: 10.1073/pnas.1105113108[69] Luxmi, Srivastava N, Feenstra R M, et al. Formation of epitaxial graphene on SiC(0001) using vacuum or argon environments. J Vac Sci Technol B, 2010, 28, C5C1 doi: 10.1116/1.3420393[70] Real M A, Lass E A, Liu F H, et al. Graphene epitaxial growth on SiC(0001) for resistance standards. IEEE Trans Instrum Meas, 2013, 62, 1454 doi: 10.1109/TIM.2012.2225962[71] Zimbone M, Zielinski M, Bongiorno C, et al. 3C-SiC growth on inverted silicon pyramids patterned substrate. Materials, 2019, 12, 3407 doi: 10.3390/ma12203407[72] Mishra N, Boeckl J, Motta N, et al. Graphene growth on silicon carbide: A review. Phys Status Solidi A, 2016, 213, 2277 doi: 10.1002/pssa.201600091[73] Yang X H, Zhang G X, Prakash J, et al. Chemical vapour deposition of graphene: Layer control, the transfer process, characterisation, and related applications. Int Rev Phys Chem, 2019, 38, 149 doi: 10.1080/0144235X.2019.1634319[74] Saeed M, Alshammari Y, Majeed S A, et al. Chemical vapour deposition of graphene—synthesis, characterisation, and applications: A review. Molecules, 2020, 25, 3856 doi: 10.3390/molecules25173856[75] Liu H T, Liu Y Q. Controlled chemical synthesis in CVD graphene. Phys Sci Rev, 2017, 2, 104 doi: 10.1515/psr-2016-0107[76] Juang Z Y, Wu C Y, Lu A Y, et al. Graphene synthesis by chemical vapor deposition and transfer by a roll-to-roll process. Carbon, 2010, 48, 3169 doi: 10.1016/j.carbon.2010.05.001[77] Alstrup I, Chorkendorff I, Ullmann S. The interaction of CH4 at high temperatures with clean and oxygen precovered Cu(100). Surf Sci, 1992, 264, 95 doi: 10.1016/0039-6028(92)90168-6[78] Hesjedal T. Continuous roll-to-roll growth of graphene films by chemical vapor deposition. Appl Phys Lett, 2011, 98, 133106 doi: 10.1063/1.3573866[79] Zhao P, Kumamoto A, Kim S, et al. Self-limiting chemical vapor deposition growth of monolayer graphene from ethanol. J Phys Chem C, 2013, 117, 10755 doi: 10.1021/jp400996s[80] Dong Y B, Guo S, Mao H H, et al. The growth of graphene on Ni–Cu alloy thin films at a low temperature and its carbon diffusion mechanism. Nanomaterials, 2019, 9, 1633 doi: 10.3390/nano9111633[81] Al-Hilfi S H, Derby B, Martin P A, et al. Chemical vapour deposition of graphene on copper-nickel alloys: The simulation of a thermodynamic and kinetic approach. Nanoscale, 2020, 12, 15283 doi: 10.1039/D0NR00302F[82] Somani P R, Somani S P, Umeno M. Planer nano-graphenes from camphor by CVD. Chem Phys Lett, 2006, 430, 56 doi: 10.1016/j.cplett.2006.06.081[83] Lee J K, Lee S, Kim Y I, et al. The seeded growth of graphene. Sci Rep, 2014, 4, 5682 doi: 10.1038/srep05682[84] Vlassiouk I, Fulvio P, Meyer H, et al. Large scale atmospheric pressure chemical vapor deposition of graphene. Carbon, 2013, 54, 58 doi: 10.1016/j.carbon.2012.11.003[85] Graphenea. Overcoming issues in CVD production of graphene. AZoNano, 2018, 3493[86] Li X S, Magnuson C W, Venugopal A, et al. Large-area graphene single crystals grown by low-pressure chemical vapor deposition of methane on copper. J Am Chem Soc, 2011, 133, 2816 doi: 10.1021/ja109793s[87] Yu Q K, Jauregui L A, Wu W, et al. Control and characterization of individual grains and grain boundaries in graphene grown by chemical vapour deposition. Nat Mater, 2011, 10, 443 doi: 10.1038/nmat3010[88] Yan Z, Lin J, Peng Z W, et al. Toward the synthesis of wafer-scale single-crystal graphene on copper foils. ACS Nano, 2012, 6, 9110 doi: 10.1021/nn303352k[89] Iwasaki T, Park H J, Konuma M, et al. Long-range ordered single-crystal graphene on high-quality heteroepitaxial Ni thin films grown on MgO(111). Nano Lett, 2011, 11, 79 doi: 10.1021/nl102834q[90] Zhang Y, Zhang L, Kim P, et al. Vapor trapping growth of single-crystalline graphene flowers: Synthesis, morphology, and electronic properties. Nano Lett, 2012, 12, 2810 doi: 10.1021/nl300039a[91] Geng D, Wu B, Guo Y, et al. Uniform hexagonal graphene flakes and films grown on liquid copper surface. PNAS, 2012, 109, 7992 doi: 10.1073/pnas.1200339109[92] Wu Y A, Fan Y, Speller S, et al. Large single crystals of graphene on melted copper using chemical vapor deposition. ACS Nano, 2012, 6, 5010 doi: 10.1021/nn3016629[93] Hao Y F, Bharathi M S, Wang L, et al. The role of surface oxygen in the growth of large single-crystal graphene on copper. Science, 2013, 342, 720 doi: 10.1126/science.1243879[94] Robertson A W, Warner J H. Hexagonal single crystal domains of few-layer graphene on copper foils. Nano Lett, 2011, 11, 1182 doi: 10.1021/nl104142k[95] Chen S, Ji H, Chou H, et al. Millimeter-size single-crystal graphene by suppressing evaporative loss of Cu during low pressure chemical vapor deposition. Adv Mater, 2013, 25, 2062 doi: 10.1002/adma.201204000[96] Polsen E S, McNerny D Q, Viswanath B, et al. High-speed roll-to-roll manufacturing of graphene using a concentric tube CVD reactor. Sci Rep, 2015, 5, 10257 doi: 10.1038/srep10257[97] Kartick B, Srivastava S K. Simple facile route for the preparation of graphite oxide and graphene. J Nanosci Nanotech, 2011, 11, 8586 doi: 10.1166/jnn.2011.4959[98] Thi Vu T H, Thi Tran T T, Thi le H N, et al. A new green approach for the reduction of graphene oxide nanosheets using caffeine. Bull Mater Sci, 2015, 38, 667 doi: 10.1007/s12034-015-0896-x[99] Esfandiar A, Akhavan O, Irajizad A. Melatonin as a powerful bio-antioxidant for reduction of graphene oxide. J Mater Chem, 2011, 21, 10907 doi: 10.1039/c1jm10151j[100] Khosroshahi Z, Kharaziha M, Karimzadeh F, et al. Green reduction of graphene oxide by ascorbic acid. AIP Conf Proc, 2018, 1920, 020009[101] Kuila T, Bose S, Khanra P, et al. A green approach for the reduction of graphene oxide by wild carrot root. Carbon, 2012, 50, 914 doi: 10.1016/j.carbon.2011.09.053[102] Li J H, Wang S S, Zhang D B, et al. Amino acids functionalized graphene oxide for enhanced hydrophilicity and antifouling property of poly(vinylidene fluoride) membranes. Chin J Polym Sci, 2016, 34, 805 doi: 10.1007/s10118-016-1808-2[103] Shankar S S, Rai A, Ahmad A, et al. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci, 2004, 275, 496 doi: 10.1016/j.jcis.2004.03.003[104] Lee G, Kim B S. Biological reduction of graphene oxide using plant leaf extracts. Biotechnol Prog, 2014, 30, 463 doi: 10.1002/btpr.1862[105] Li Y, Wu Y. Coassembly of graphene oxide and nanowires for large-area nanowire alignment. J Am Chem Soc, 2009, 131, 5851 doi: 10.1021/ja9000882[106] Zhu X, Xu X L, Liu F, et al. Green synthesis of graphene nanosheets and their in vitro cytotoxicity against human prostate cancer (DU 145) cell lines. Nanomater Nanotechnol, 201, 7, 184798041770279 doi: 10.1177/1847980417702794[107] Perera S D, Mariano R G, Nijem N, et al. Alkaline deoxygenated graphene oxide for supercapacitor applications: An effective green alternative for chemically reduced graphene. J Power Sources, 2012, 215, 1 doi: 10.1016/j.jpowsour.2012.04.059[108] de Silva K K H, Huang H H, Yoshimura M. Progress of reduction of graphene oxide by ascorbic acid. Appl Surf Sci, 2018, 447, 338 doi: 10.1016/j.apsusc.2018.03.243[109] Tavakoli F, Salavati-Niasari M, badiei A, et al. Green synthesis and characterization of graphene nanosheets. Mater Res Bull, 2015, 63, 51 doi: 10.1016/j.materresbull.2014.11.045[110] Akbar S A, Nanda F, Mawaddah N, et al. Green synthesis of reduced graphene oxide using lime juice reductor from citrus aurantifolia. Elkawnie, 2019, 5, 139 doi: 10.22373/ekw.v5i2.4948[111] Kumawat M K, Thakur M, Gurung R B, et al. Graphene quantum dots from mangifera indica: Application in near-infrared bioimaging and intracellular nanothermometry. ACS Sustain Chem Eng, 2017, 5, 1382 doi: 10.1021/acssuschemeng.6b01893[112] Wu R Z, Ding Y, Yu K M, et al. Edge-epitaxial growth of graphene on Cu with a hydrogen-free approach. Chem Mater, 2019, 31, 2555 doi: 10.1021/acs.chemmater.9b00147[113] Dong J, Zhang L, Dai X, et al. The epitaxy of 2D materials growth. Nat Commun, 2020, 11, 5862 doi: 10.1038/s41467-020-19752-3[114] Huang H, Chen S, Wee A T, et al. Epitaxial growth of graphene on silicon carbide (SiC). In: Graphene. Woodhead Publishing, 2014[115] Moreno-Bárcenas A, Perez-Robles J F, Vorobiev Y V, et al. Graphene synthesis using a CVD reactor and a discontinuous feed of gas precursor at atmospheric pressure. J Nanomater, 2018, 2018, 3457263 doi: 10.1155/2018/3457263[116] Smovzh D V, Kostogrud I A, Boyko E V, et al. Synthesis of graphene by chemical vapor deposition and its transfer to polymer. J Appl Mech Tech Phy, 2020, 61, 888 doi: 10.1134/S0021894420050247[117] Meka Chufa B, Abdisa Gonfa B, Yohannes Anshebo T, et al. A novel and simplest green synthesis method of reduced graphene oxide using methanol extracted Vernonia amygdalina: Large-scale production. Adv Condens Matter Phys, 2021, 2021, 6681710 doi: 10.1155/2021/6681710[118] Bhattacharya G, Sas S, Wadhwa S, et al. Aloe vera assisted facile green synthesis of reduced graphene oxide for electrochemical and dye removal applications. RSC Adv, 2017, 7, 26680 doi: 10.1039/C7RA02828H[119] Salunke B K, Kim B S. Facile synthesis of graphene using a biological method. RSC Adv, 2016, 6, 17158 doi: 10.1039/C5RA25977K[120] Pirzado A, Le Normand F, Romero T, et al. Few-layer graphene from mechanical exfoliation of graphite-based materials: Structure-dependent characteristics. ChemEngineering, 2019, 3, 37 doi: 10.3390/chemengineering3020037[121] Jayasena B, Melkote S N. An investigation of PDMS stamp assisted mechanical exfoliation of large area graphene. Procedia Manuf, 2015, 1, 840 doi: 10.1016/j.promfg.2015.09.073[122] Danial W H, Norhisham N A, Ahmad Noorden A F, et al. A short review on electrochemical exfoliation of graphene and graphene quantum dots. Carbon Lett, 2021, 31, 371 doi: 10.1007/s42823-020-00212-3[123] Ahirwar S, Mallick S, Bahadur D. Electrochemical method to prepare graphene quantum dots and graphene oxide quantum dots. ACS Omega, 2017, 2, 8343 doi: 10.1021/acsomega.7b01539 -
Proportional views






 DownLoad:
DownLoad: