| Citation: |
Banpeng Cao, Changhao Dai, Xuejun Wang, Dacheng Wei. Ultrasensitive detection of methamphetamine by antibody-modified transistor assay[J]. Journal of Semiconductors, 2023, 44(2): 022001. doi: 10.1088/1674-4926/44/2/022001
****
B P Cao, C H Dai, X J Wang, D C Wei. Ultrasensitive detection of methamphetamine by antibody-modified transistor assay[J]. J. Semicond, 2023, 44(2): 022001. doi: 10.1088/1674-4926/44/2/022001
|
Ultrasensitive detection of methamphetamine by antibody-modified transistor assay
DOI: 10.1088/1674-4926/44/2/022001
More Information
-
Abstract
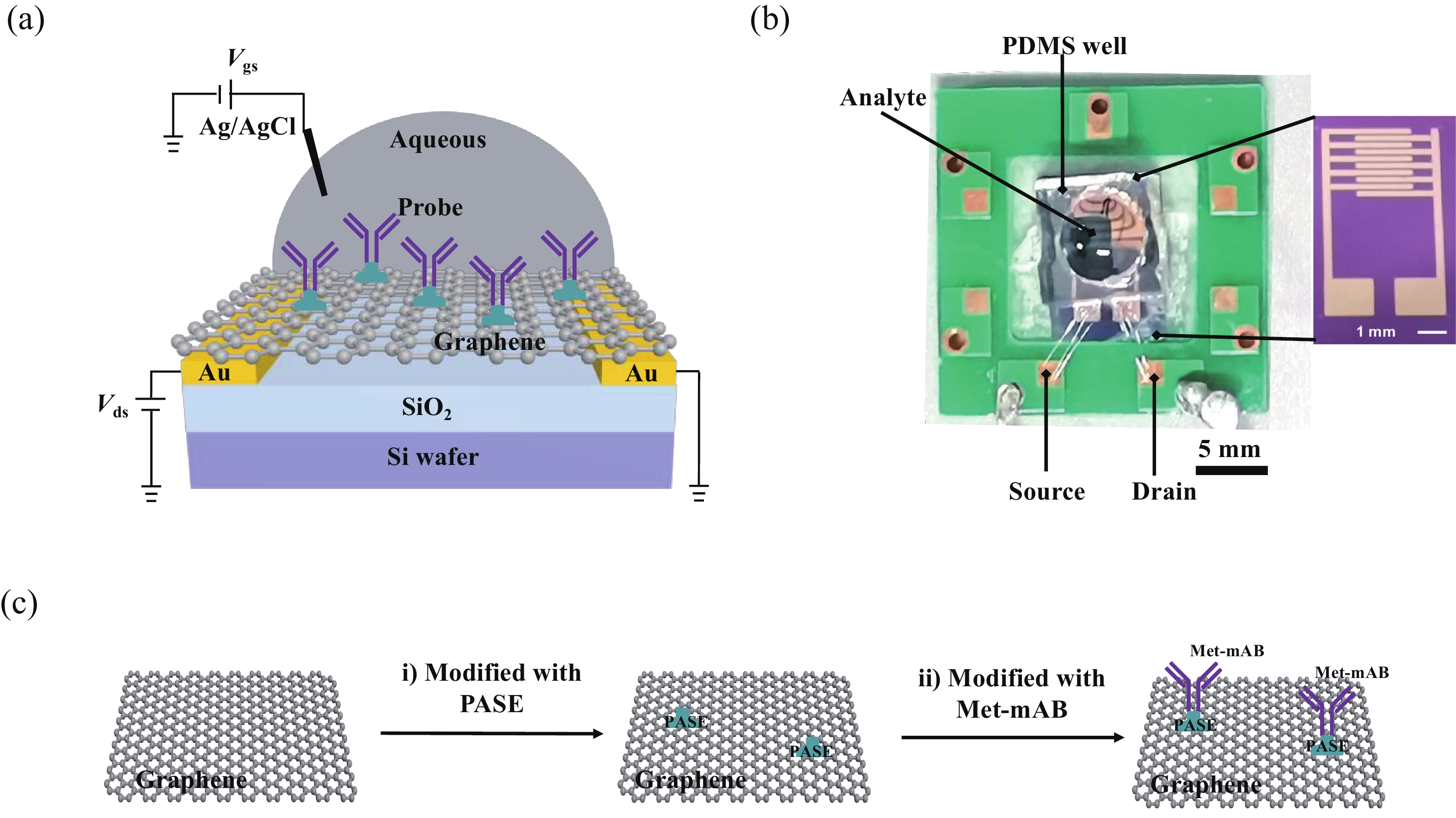
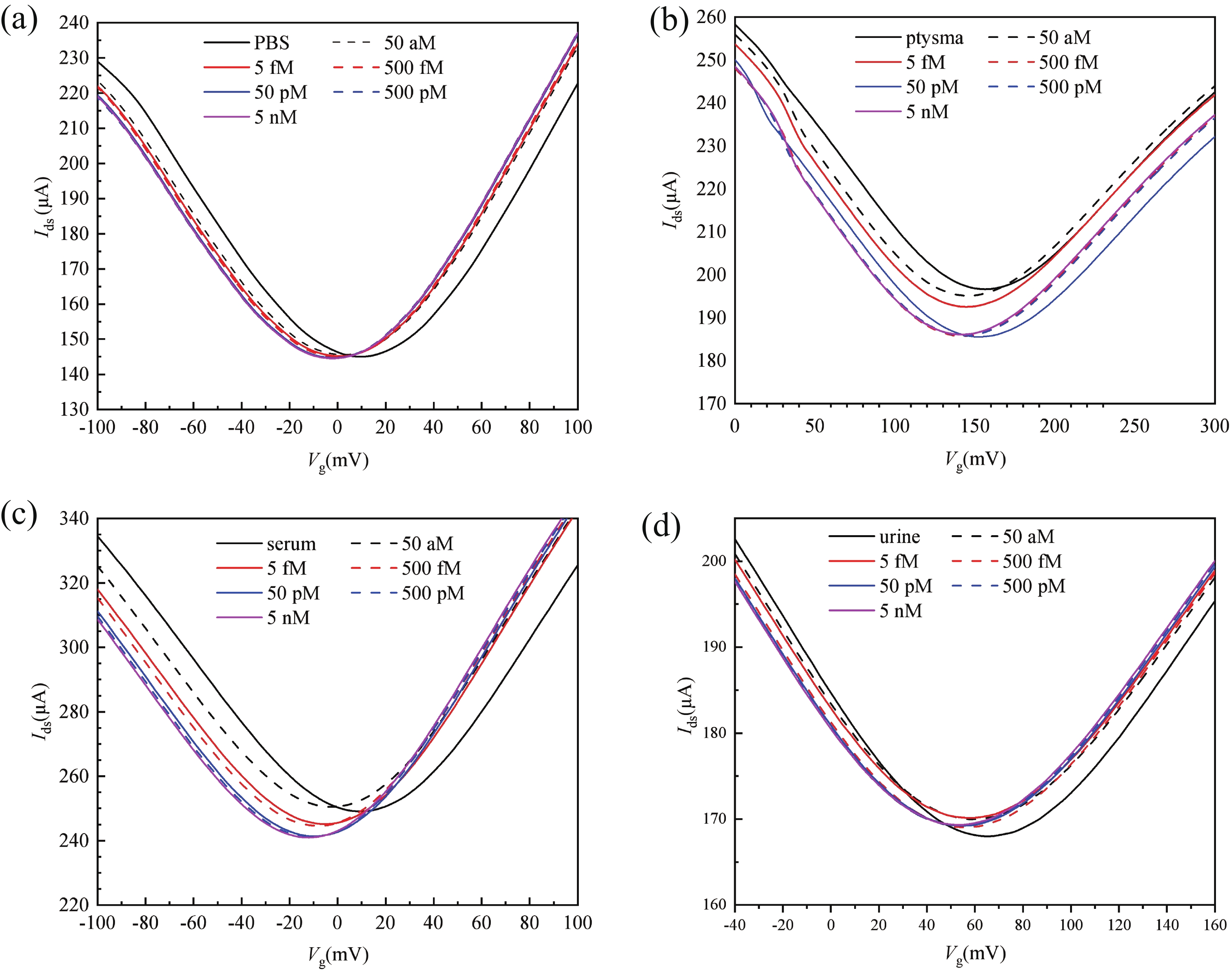
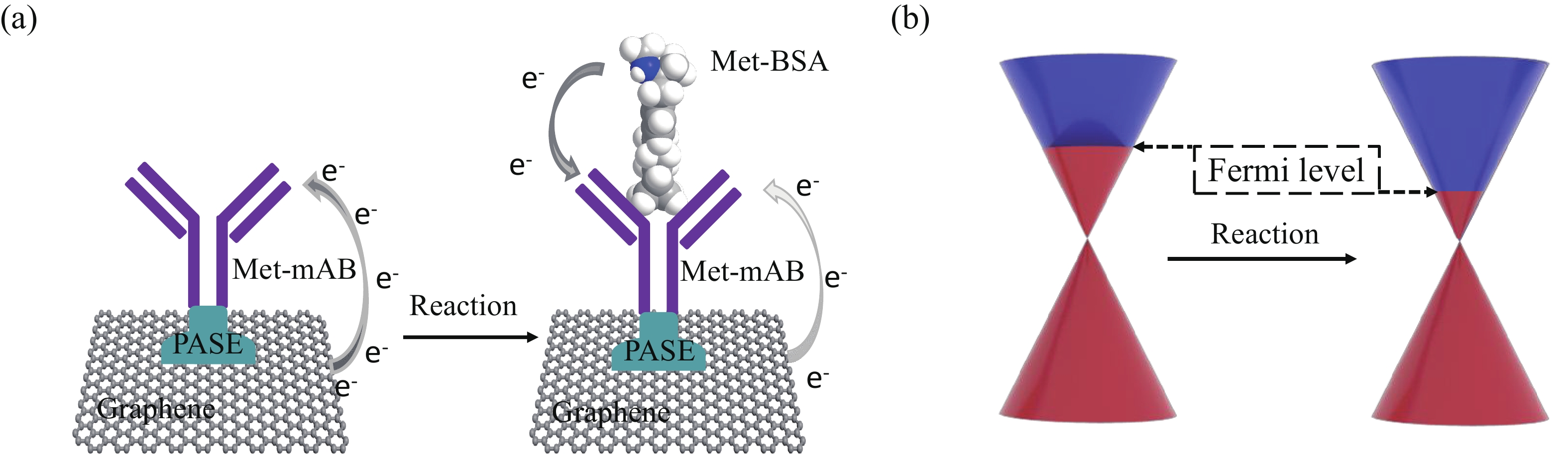
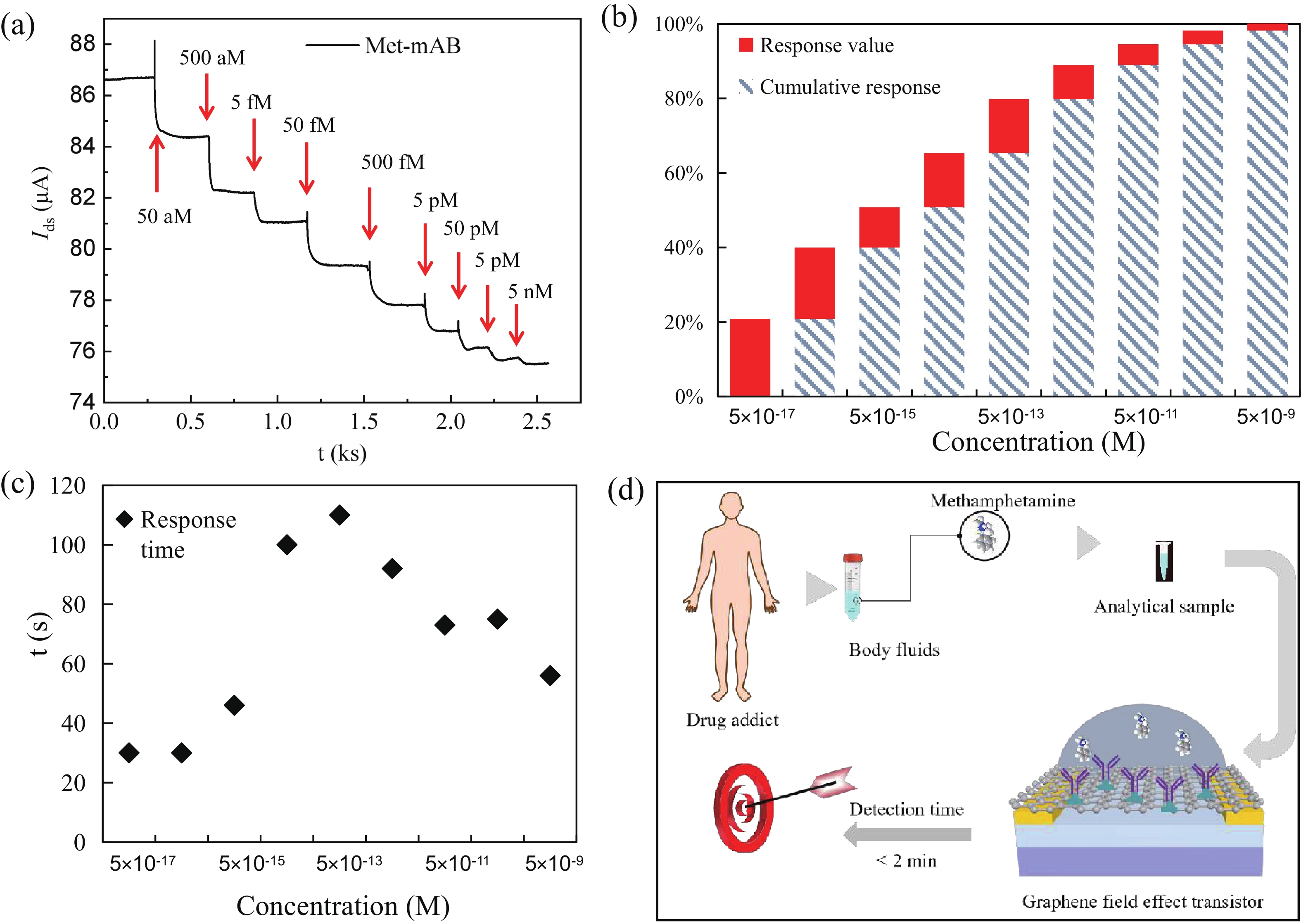
Effective detection of methamphetamine (Met) requires a fast, sensitive, and cheap testing assay. However, commercially available methods require expensive instruments and highly trained operators, which are time-consuming and labor-intensive. Herein, an antibody-modified graphene transistor assay is developed for sensitive and minute-level detection of Met in complex environments. The anti-Met probe captured charged targets within 120 s, leading to a p-doping effect near the graphene channel. The limit of detection reaches 50 aM (5.0 × 10−17 M) Met in solution. The graphene transistor would be a valuable tool for Met detection effective prevention of drug abuse. -
References
[1] Chang T C, Chiang C Y, Lin M H, et al. Fiber optic particle plasmon resonance immunosensor for rapid and sensitive detection of methamphetamine based on competitive inhibition. Microchem J, 2020, 157, 105026 doi: 10.1016/j.microc.2020.105026[2] Kumar A, Dangi I, Pawar R. Drug addiction: A big challenge for youth and children's. Int J Res Pharm Pharm Sci Internet, 2019, 4, 35[3] Harastani M, Benterkia A, Zadeh F M, et al. Methamphetamine drug abuse and addiction: Effects on face asymmetry. Comput Biol Med, 2020, 116, 103475 doi: 10.1016/j.compbiomed.2019.103475[4] Wu K R, Hsiao H H. Rapid and accurate quantification of amphetamine and methamphetamine in human urine by antibody decorated magnetite nanoparticles coupled with matrix-assisted laser desorption ionization time-of-flight mass spectrometer analysis. Anal Chimica Acta, 2018, 1025, 134 doi: 10.1016/j.aca.2018.03.057[5] Bor G, Bulut U, Man E, et al. Synthetic antibodies for methamphetamine analysis: Design of high affinity aptamers and their use in electrochemical biosensors. J Electroanal Chem, 2022, 921, 116686 doi: 10.1016/j.jelechem.2022.116686[6] Ghorbanizamani F, et al. Ionic liquid-hydrogel hybrid material for enhanced electron transfer and sensitivity towards electrochemical detection of methamphetamine. J Mol Liq, 2022, 361, 119627 doi: 10.1016/j.molliq.2022.119627[7] Dai C H, Liu Y Q, Wei D C. Two-dimensional field-effect transistor sensors: The Road toward commercialization. Chem Rev, 2022, 122, 10319 doi: 10.1021/acs.chemrev.1c00924[8] Abbasi H Y, Tehrani Z, Devadoss A, et al. Graphene based electrochemical immunosensor for the ultra-sensitive label free detection of Alzheimer's beta amyloid peptides Aβ(1-42). Nanoscale Adv, 2021, 3, 2295 doi: 10.1039/D0NA00801J[9] Wang R R, Mao Y, Wang L, et al. Solution-gated graphene transistor based sensor for histamine detection with gold nanoparticles decorated graphene and multi-walled carbon nanotube functionalized gate electrodes. Food Chem, 2021, 347, 128980 doi: 10.1016/j.foodchem.2020.128980[10] Kang H, Wang X J, Guo M Q, et al. Ultrasensitive detection of SARS-CoV-2 antibody by graphene field-effect transistors. Nano Lett, 2021, 21, 7897 doi: 10.1021/acs.nanolett.1c00837[11] Forsyth R, Devadoss A, Guy O J. Graphene field effect transistors for biomedical applications: Current status and future prospects. Diagnostics, 2017, 7, 45 doi: 10.3390/diagnostics7030045[12] Fu W, El Abbassi M, Hasler T, et al. Electrolyte gate dependent high-frequency measurement of graphene field-effect transistor for sensing applications. Appl Phys Lett, 2014, 104, 013102 doi: 10.1063/1.4857616[13] Zhan B B, Li C, Yang J, et al. Graphene field-effect transistor and its application for electronic sensing. Small, 2014, 10, 4042 doi: 10.1002/smll.201400463[14] Nag A, Mitra A, Mukhopadhyay S C. Graphene and its sensor-based applications: A review. Sens Actuat A, 2018, 270, 177 doi: 10.1016/j.sna.2017.12.028[15] Wang L Q, Wang X J, Wu Y G, et al. Rapid and ultrasensitive electromechanical detection of ions, biomolecules and SARS-CoV-2 RNA in unamplified samples. Nat Biomed Eng, 2022, 6, 276 doi: 10.1038/s41551-021-00833-7[16] Jang Y Jang M, Kim H, et al. Point-of-use detection of amphetamine-type stimulants with host-molecule-functionalized organic transistors. Chem, 2017, 3, 641 doi: 10.1016/j.chempr.2017.08.015[17] Du X C, Hao H X, Qin A J, et al. Highly sensitive chemosensor for detection of methamphetamine by the combination of AIE luminogen and cucurbit[7]uril. Dyes Pigments, 2020, 180, 108413 doi: 10.1016/j.dyepig.2020.108413[18] Mandani S, Rezaei B, Ensafi A A. Sensitive imprinted optical sensor based on mesoporous structure and green nanoparticles for the detection of methamphetamine in plasma and urine. Spectrochim Acta A, 2020, 231, 118077 doi: 10.1016/j.saa.2020.118077[19] Ganapati S, Isaacs L. Acyclic cucurbit[n]uril-type receptors: Preparation, molecular recognition properties and biological applications. Isr J Chem, 2018, 58, 250 doi: 10.1002/ijch.201700098[20] Dai C H, Guo M Q, Wu Y L, et al. Ultraprecise antigen 10-in-1 pool testing by multiantibodies transistor assay. J Am Chem Soc, 2021, 143, 19794 doi: 10.1021/jacs.1c08598[21] Yang Y B, Yang X D, Zou X M, et al. Ultrafine graphene nanomesh with large on/off ratio for high-performance flexible biosensors. Adv Funct Mater, 2017, 27, 1604096 doi: 10.1002/adfm.201604096[22] Song Y Q, Zou W T, Lu Q, et al. Graphene transfer: Paving the road for applications of chemical vapor deposition graphene. Small, 2021, 17, 2007600 doi: 10.1002/smll.202007600 -
Supplements
 22100025suppl.pdf
22100025suppl.pdf

-
Proportional views

§Banpeng Cao and Changhao Dai contributed equally to this work.




 DownLoad:
DownLoad:










 Banpeng Cao:received his master degree from Jiangxi Normal University, Jiangxi, China in 2011 and PhD from the Yamaguchi University, Yamaguchi, Japan in 2017. He joined Dacheng Wei's Group at Fudan University as a postdoc researcher in June 2019. His research interests center on preparation of 2D materials and their sensing applications
Banpeng Cao:received his master degree from Jiangxi Normal University, Jiangxi, China in 2011 and PhD from the Yamaguchi University, Yamaguchi, Japan in 2017. He joined Dacheng Wei's Group at Fudan University as a postdoc researcher in June 2019. His research interests center on preparation of 2D materials and their sensing applications Changhao Dai:was born in 1997. He received his bachelor degree from Nanjing University of Science and Technology in 2019. He is currently pursuing a PhD at the Department of Macromolecular Science, Fudan University at Shanghai, China, under the guidance of Prof. Dacheng Wei. His research interests center on preparation of 2D materials and their sensing applications
Changhao Dai:was born in 1997. He received his bachelor degree from Nanjing University of Science and Technology in 2019. He is currently pursuing a PhD at the Department of Macromolecular Science, Fudan University at Shanghai, China, under the guidance of Prof. Dacheng Wei. His research interests center on preparation of 2D materials and their sensing applications Dacheng Wei:is a professor in the Department of Marcomolecular Science, Fudan University, Shanghai, China. He received his B.S. degree from Zhejiang University, Zhejiang, China in 2003 and PhD from the Institute of Chemistry, Chinese Academy of Sciences, Beijing, China in 2009. After his independent research in National University of Singapore, Singapore, he joined Fudan University in 2014. His research interest includes synthesis and electrical applications of 2D materials like graphene, 2D polymers, and so on
Dacheng Wei:is a professor in the Department of Marcomolecular Science, Fudan University, Shanghai, China. He received his B.S. degree from Zhejiang University, Zhejiang, China in 2003 and PhD from the Institute of Chemistry, Chinese Academy of Sciences, Beijing, China in 2009. After his independent research in National University of Singapore, Singapore, he joined Fudan University in 2014. His research interest includes synthesis and electrical applications of 2D materials like graphene, 2D polymers, and so on





