| Citation: |
Ling Yang, Yizheng Huang, Zhigang Song, Manqing Tan, Yude Yu, Zhao Li. A 640 × 640 ISFET array for detecting cell metabolism[J]. Journal of Semiconductors, 2023, 44(2): 024101. doi: 10.1088/1674-4926/44/2/024101
****
L Yang, Y Z Huang, Z G Song, M Q Tan, Y D Yu, Z Li. A 640 × 640 ISFET array for detecting cell metabolism[J]. J. Semicond, 2023, 44(2): 024101. doi: 10.1088/1674-4926/44/2/024101
|
A 640 × 640 ISFET array for detecting cell metabolism
DOI: 10.1088/1674-4926/44/2/024101
More Information
-
Abstract
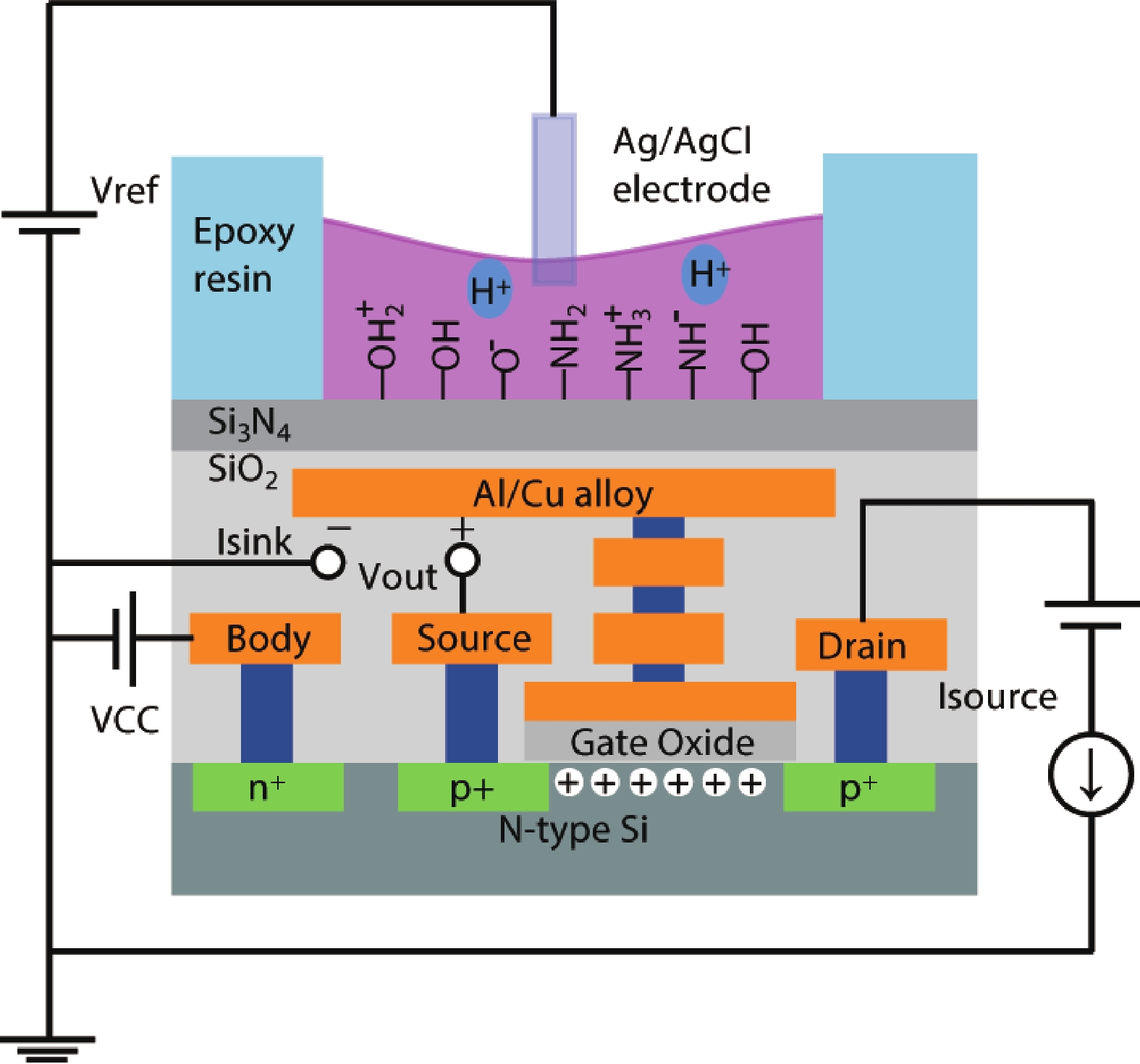
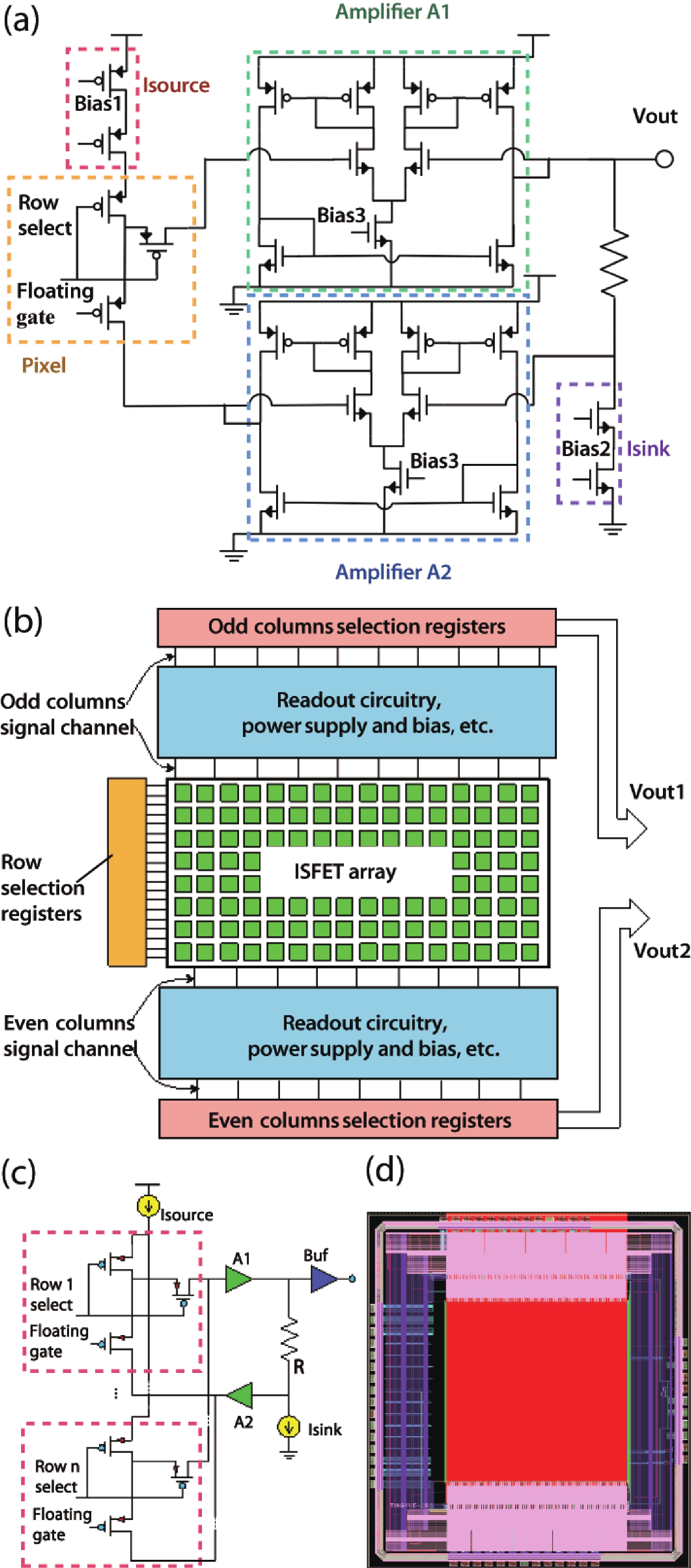
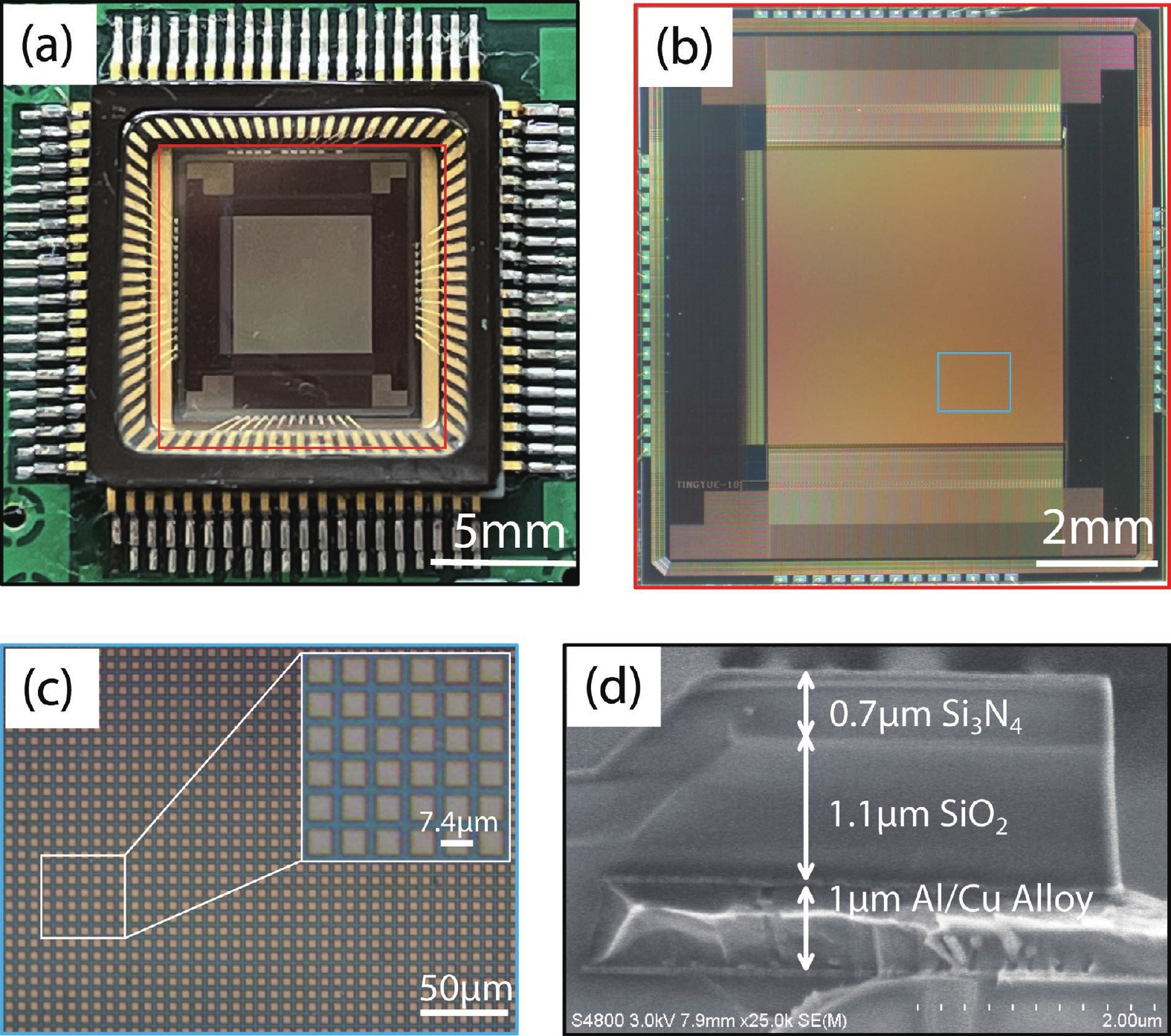
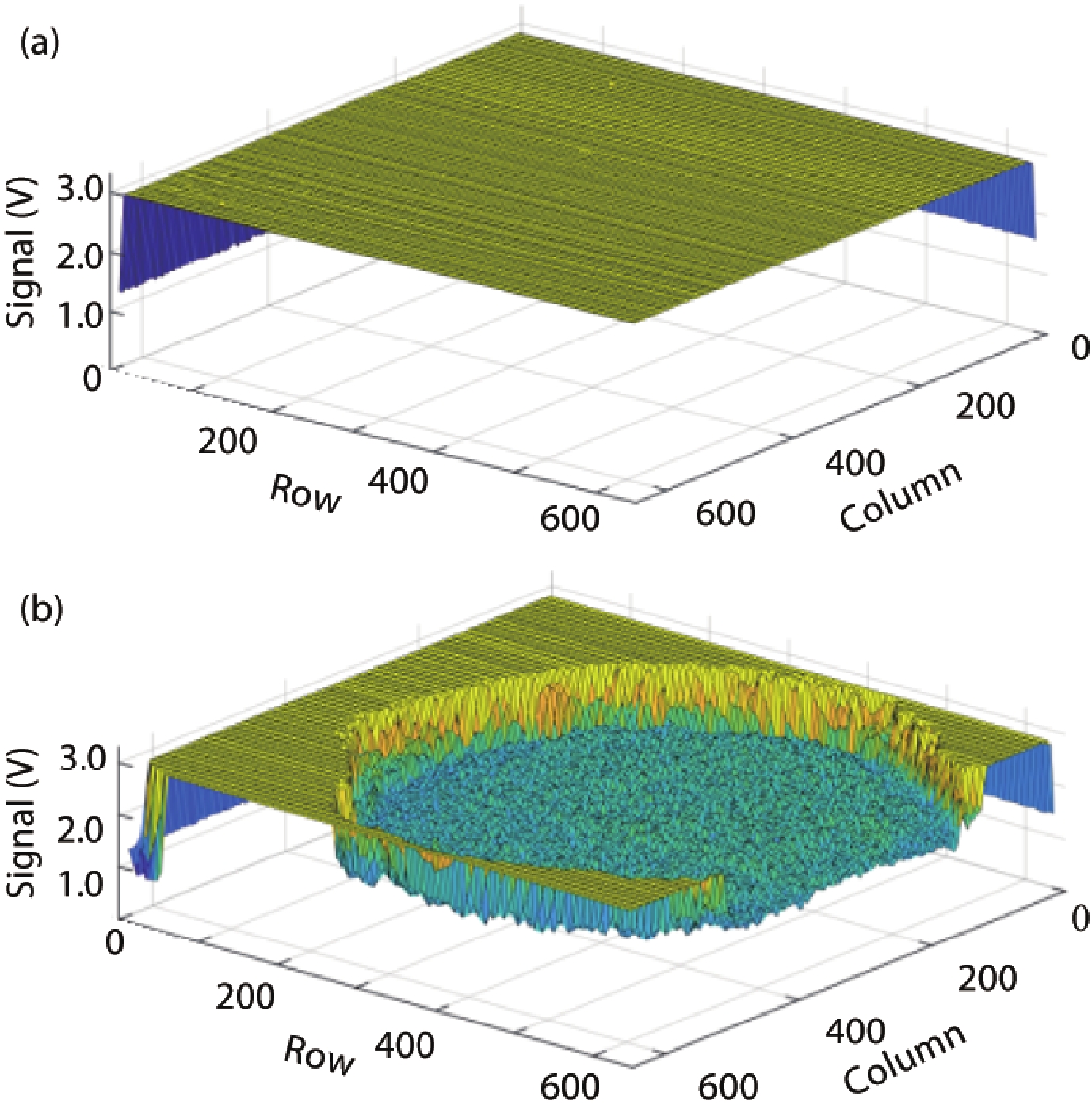
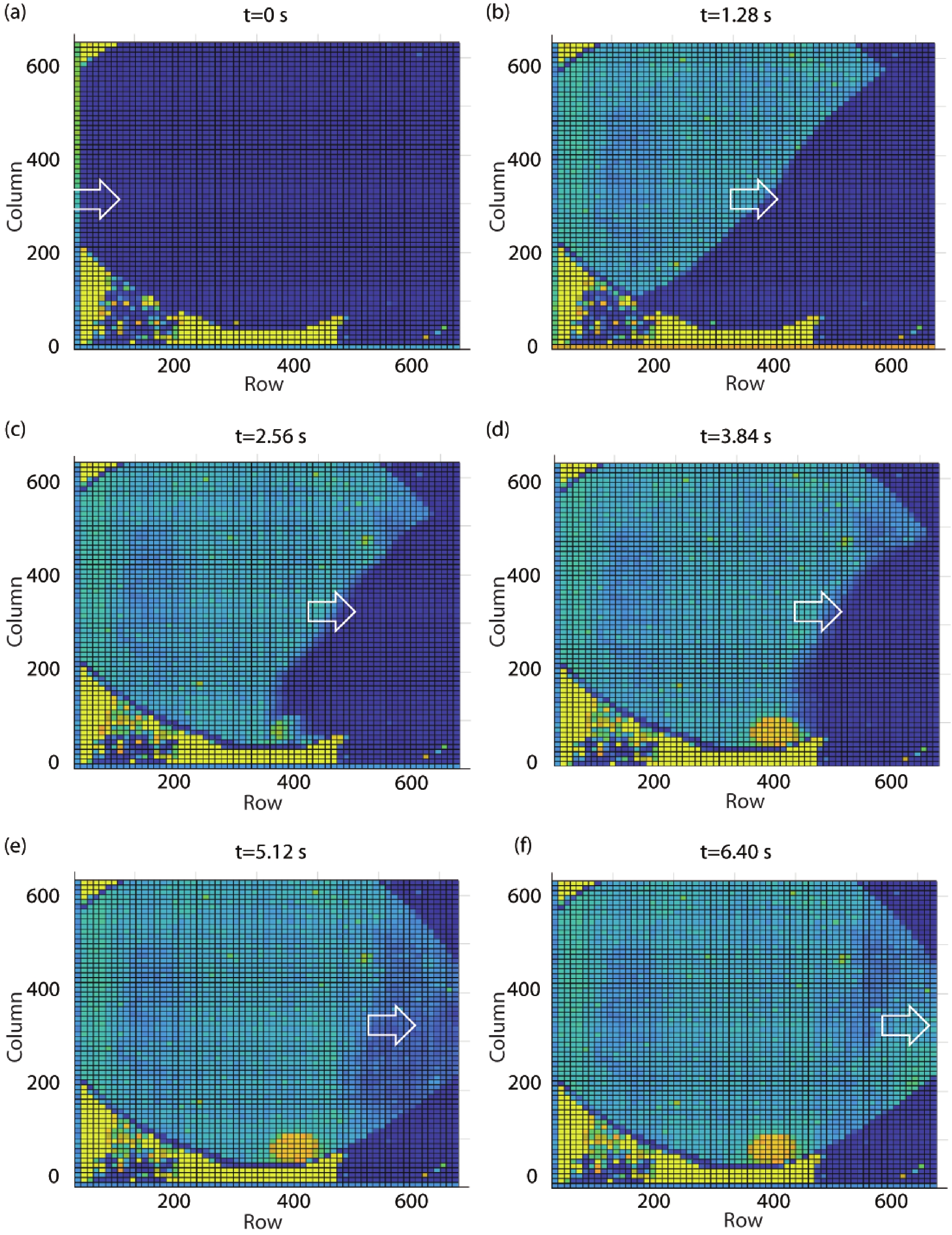
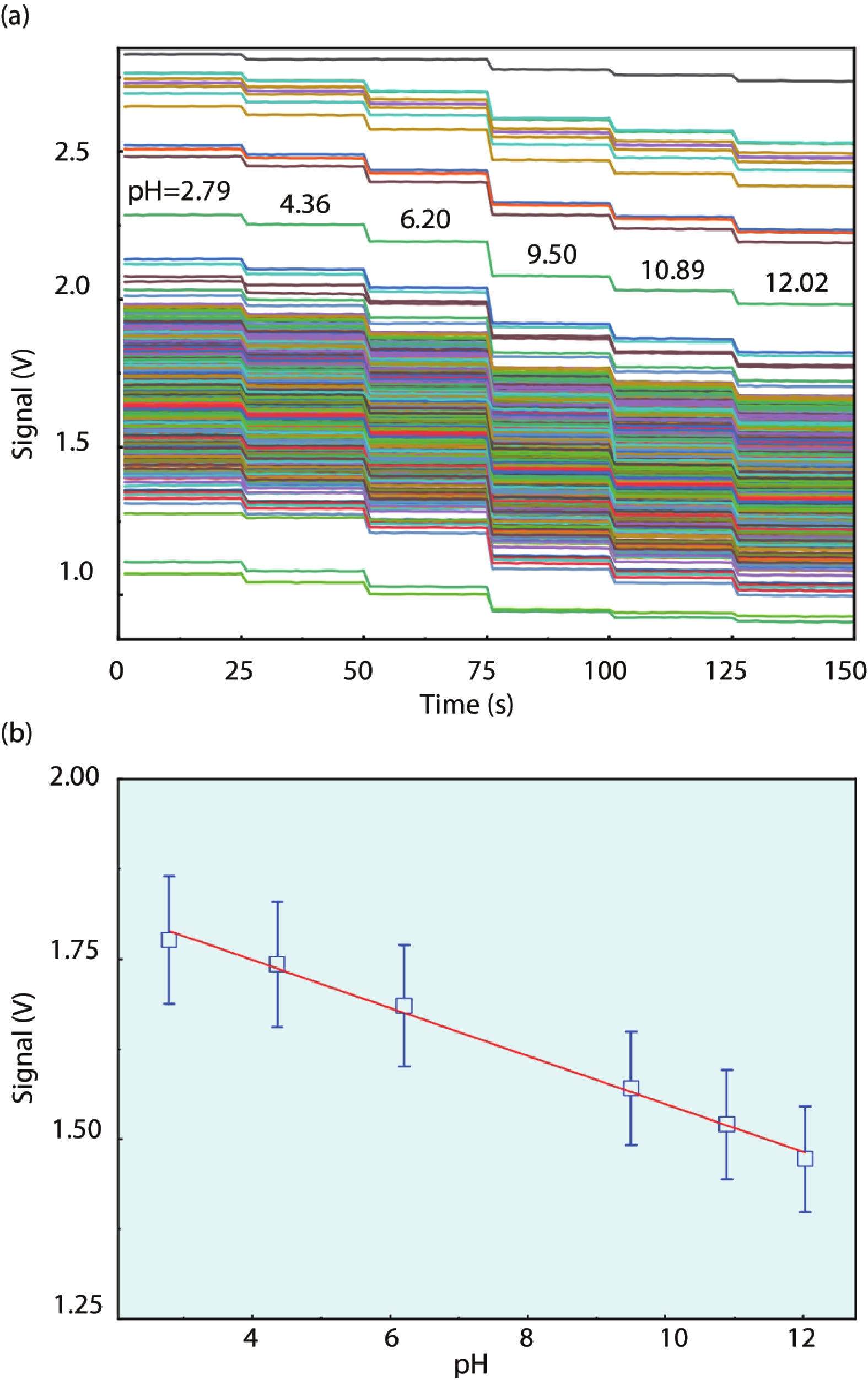
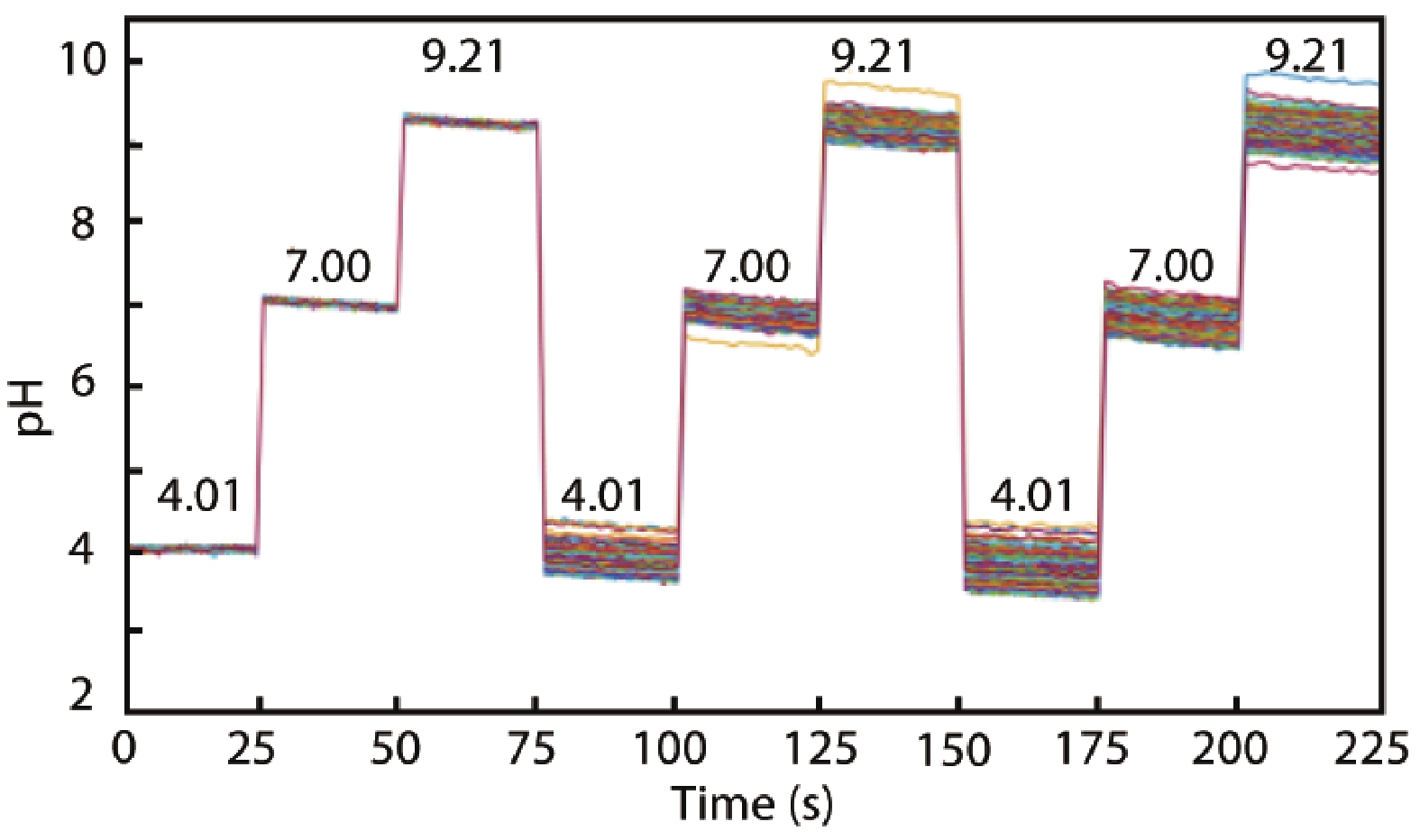
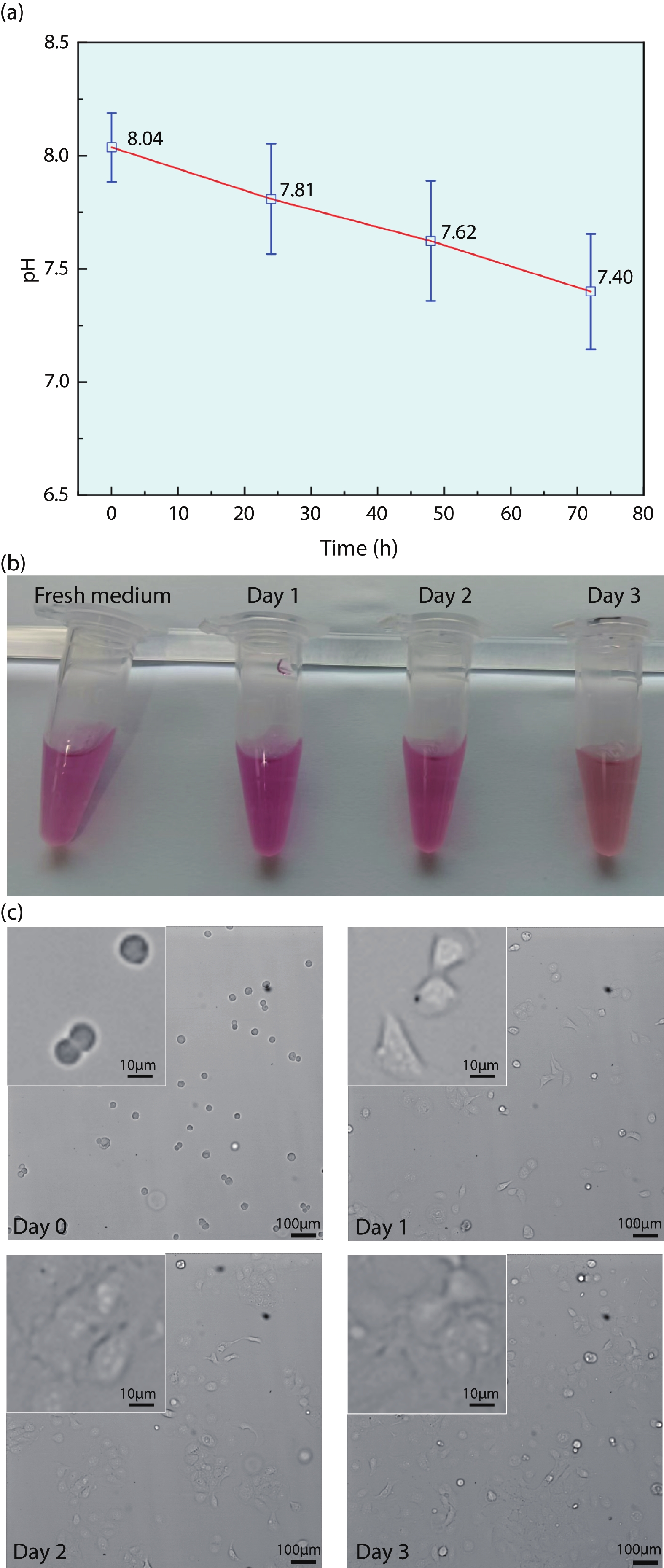
Ion sensitive field effect transistor (ISFET) devices are highly accurate, convenient, fast and low-cost in the detection of ions and biological macromolecules, such as DNA molecules, antibodies, enzymatic substrates and cellular metabolites. For high-throughput cell metabolism detection, we successfully designed a very large-scale biomedical sensing application specific integrated circuit (ASIC) with a 640 × 640 ISFET array. The circuit design is highly integrated by compressing the size of a pixel to 7.4 × 7.4 μm2 and arranging the layout of even and odd columns in an interdigital pattern to maximize the utilization of space. The chip can operate at a speed of 2.083M pixels/s and the dynamic process of the fluid flow on the surface of the array was monitored through ion imaging. The pH sensitivity is 33 ± 4 mV/pH and the drift rate is 0.06 mV/min after 5 h, indicating the stability and robustness of the chip. Moreover, the chip was applied to monitor pH changes in CaSki cells metabolism, with pH shifting from 8.04 to 7.40 on average. This platform has the potential for continuous and parallel monitoring of cell metabolism in single-cell culture arrays.-
Keywords:
- ASIC,
- ISFET array,
- pH monitoring,
- ion imaging,
- cell metabolism
-
References
[1] DeBerardinis R J, Thompson C B. Cellular metabolism and disease: What do metabolic outliers teach us? Cell, 2012, 148, 1132 doi: 10.1016/j.cell.2012.02.032[2] Solaini G, Sgarbi G, Baracca A. Oxidative phosphorylation in cancer cells. Biochim Biophys Acta, 2011, 1807, 534 doi: 10.1016/j.bbabio.2010.09.003[3] Wang J X, Choi S Y C, Niu X J, et al. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci, 2020, 21, 8363 doi: 10.3390/ijms21218363[4] Thews O, Riemann A. Tumor pH and metastasis: A malignant process beyond hypoxia. Cancer Metastasis Rev, 2019, 38, 113 doi: 10.1007/s10555-018-09777-y[5] Rueck A C, Hauser C, Mosch S, et al. Spectrally resolved fluorescence lifetime imaging to investigate cell metabolism in malignant and nonmalignant oral mucosa cells. J Biomed Opt, 2014, 19, 096005 doi: 10.1117/1.JBO.19.9.096005[6] Le Guern F, Mussard V, Gaucher A, et al. Fluorescein derivatives as fluorescent probes for pH monitoring along recent biological applications. Int J Mol Sci, 2020, 21, 9217 doi: 10.3390/ijms21239217[7] Fatemeh S, Soheil J, Abdolhosein H, et al. Effects of variations of voltage and pH value on the shear strength of soil and durability of different electrodes and piles during electrokinetic phenomenon. J Rock Mech Geotech Eng, 2022, 14, 625 doi: 10.1016/j.jrmge.2021.07.017[8] Tao Z, Si H W, Zhang X D, et al. Highly sensitive and selective H2O2 sensors based on ZnO TFT using PBNCs/Pt-NPs/TNTAs as gate electrode. Sens Actuat B, 2021, 349, 130791 doi: 10.1016/j.snb.2021.130791[9] Bergveld P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans Biomed Eng, 1970, BME-17, 70 doi: 10.1109/TBME.1970.4502688[10] Bergveld P. Thirty years of ISFETOLOGY: what happened in the past 30 years and what may happen in the next 30 years. Sens Actuat B, 2003, 88, 1 doi: 10.1016/S0925-4005(02)00301-5[11] Cao S L, Sun P, Xiao G, et al. ISFET-based sensors for (bio)chemical applications: A review. Electrochem Sci Adv, 2022, 2100207 doi: 10.1002/elsa.202100207[12] Douthwaite M, Moser N, Georgiou P. CMOS ISFET arrays for integrated electrochemical sensing and imaging applications: A tutorial. IEEE Sens J, 2021, 21, 22155 doi: 10.1109/JSEN.2021.3094206[13] Rothberg J M, Hinz W, Rearick T M, et al. An integrated semiconductor device enabling non-optical genome sequencing. Nature, 2011, 475, 348 doi: 10.1038/nature10242[14] Sun P, Cong Y X, Xu M, et al. An ISFET microarray sensor system for detecting the DNA base pairing. Micromachines, 2021, 12, 731 doi: 10.3390/mi12070731[15] Toumazou C, Shepherd L M, Reed S C, et al. Simultaneous DNA amplification and detection using a pH-sensing semiconductor system. Nat Methods, 2013, 10, 641 doi: 10.1038/nmeth.2520[16] Cacho-Soblechero M, Malpartida-Cardenas K, Cicatiello C, et al. A dual-sensing thermo-chemical ISFET array for DNA-based diagnostics. IEEE Trans Biomed Circuits Syst, 2020, 14, 477 doi: 10.1109/TBCAS.2020.2978000[17] Duan M Z, Zhong X P, Xu J T, et al. A high offset distribution tolerance high resolution ISFET array with auto-compensation for long-term bacterial metabolism monitoring. IEEE Trans Biomed Circuits Syst, 2020, 14, 463 doi: 10.1109/TBCAS.2020.2977960[18] Yang L H, Liu X B, Yin B, et al. High-throughput and real-time monitoring of single-cell extracellular pH based on polyaniline microarrays. Anal Chem, 2021, 93, 13852 doi: 10.1021/acs.analchem.1c02560[19] Milgrew M J, et al. The development of scalable sensor arrays using standard CMOS technology. Sens Actuat B, 2004, 103, 37 doi: 10.1016/j.snb.2004.03.004[20] van Hal R E G, et al. A general model to describe the electrostatic potential at electrolyte oxide interfaces. Adv Colloid Interface Sci, 1996, 69, 31 doi: 10.1016/S0001-8686(96)00307-7[21] Yang L, et al. A high-speed small-area pixel 16 × 16 ISFET array design using 0.35-μm CMOS process. Microelectron J, 2018, 79, 107 doi: 10.1016/j.mejo.2018.03.003[22] Nakazato K. An integrated ISFET sensor array. Sensors, 2009, 9, 8831 doi: 10.3390/s91108831[23] Miscourides N, Georgiou P. Calibrating for trapped charge in large-scale ISFET arrays. IEEE Sens J, 2020, 20, 5110 doi: 10.1109/JSEN.2020.2968578[24] Miscourides N, Yu L S, Rodriguez-Manzano J, et al. A 12.8 k current-mode velocity-saturation ISFET array for on-chip real-time DNA detection. IEEE Trans Biomed Circuits Syst, 2018, 12, 1202 doi: 10.1109/TBCAS.2018.2851448[25] Moser N, Rodriguez-Manzano J, Lande T S, et al. A scalable ISFET sensing and memory array with sensor auto-calibration for on-chip real-time DNA detection. IEEE Trans Biomed Circuits Syst, 2018, 12, 390 doi: 10.1109/TBCAS.2017.2789161[26] Sakata T, Sugimoto H, Saito A. Live monitoring of microenvironmental pH based on extracellular acidosis around cancer cells with cell-coupled gate ion-sensitive field-effect transistor. Anal Chem, 2018, 90, 12731 doi: 10.1021/acs.analchem.8b03070 -
Proportional views





 DownLoad:
DownLoad:










 Ling Yang:received his B.Eng. degree in Microelectronics from Northwestern Polytechnical University, Xi’an, China, and the Ph.D. Degree from Institute of Semiconductors, Chinese Academy of Sciences, in 2013 and 2018, respectively. He is currently working as a Postdoctoral Researcher at the Optoelectronics R&D Center, Institute of Semiconductors, Chinese Academy of Sciences, for development of DNA sequencing machine based on ISFET technology and cell separation chip based on DEP technology
Ling Yang:received his B.Eng. degree in Microelectronics from Northwestern Polytechnical University, Xi’an, China, and the Ph.D. Degree from Institute of Semiconductors, Chinese Academy of Sciences, in 2013 and 2018, respectively. He is currently working as a Postdoctoral Researcher at the Optoelectronics R&D Center, Institute of Semiconductors, Chinese Academy of Sciences, for development of DNA sequencing machine based on ISFET technology and cell separation chip based on DEP technology Yizheng Huang:received her B.S. degree from Beijing Jiaotong University in 2016. Now she is currently a Ph.D. candidate under the supervision of Professor Yude Yu at Institute of Semiconductors, Chinese Academy of Sciences. Her current research focuses on microfluidic techniques, cell analysis and biosensing
Yizheng Huang:received her B.S. degree from Beijing Jiaotong University in 2016. Now she is currently a Ph.D. candidate under the supervision of Professor Yude Yu at Institute of Semiconductors, Chinese Academy of Sciences. Her current research focuses on microfluidic techniques, cell analysis and biosensing Yude Yu:received his B.Eng. degree from the Chinese University of Sciences and Technology. He got his Ph.D. degree from the Institute of inorganic Chemistry, Russia Academy of Sciences. He has since worked in space material research, integrated photonics devices and systems, and nerve and muscle impedance spectrometry. Currently his main interest is in the development of the next generation of gene sequencing technology, dPCR, high precision single cell in situ gene analysis, and portable biosensing devices
Yude Yu:received his B.Eng. degree from the Chinese University of Sciences and Technology. He got his Ph.D. degree from the Institute of inorganic Chemistry, Russia Academy of Sciences. He has since worked in space material research, integrated photonics devices and systems, and nerve and muscle impedance spectrometry. Currently his main interest is in the development of the next generation of gene sequencing technology, dPCR, high precision single cell in situ gene analysis, and portable biosensing devices Zhao Li:received his B.Eng. degree in Communication Engineering from Wuhan University of Technology in 2012, and the Ph.D. Degree in Physical Electronics from the University of Chinese Academy of Sciences in 2017. From 2017 to 2021, he worked as a postdoctoral researcher in Ping Wang's lab at the Perelman School of Medicine, University of Pennsylvania. His research interests focus on developing and validating of innovative point of care devices with novel biosensors and microfluidics. Since 2021, he has been with the Institute of Semiconductors, Chinese Academy of Sciences, as a full professor working on the novel biochemical sensors and medical testing equipment development
Zhao Li:received his B.Eng. degree in Communication Engineering from Wuhan University of Technology in 2012, and the Ph.D. Degree in Physical Electronics from the University of Chinese Academy of Sciences in 2017. From 2017 to 2021, he worked as a postdoctoral researcher in Ping Wang's lab at the Perelman School of Medicine, University of Pennsylvania. His research interests focus on developing and validating of innovative point of care devices with novel biosensors and microfluidics. Since 2021, he has been with the Institute of Semiconductors, Chinese Academy of Sciences, as a full professor working on the novel biochemical sensors and medical testing equipment development



