| Citation: |
Zhimin Fang, Jie Sun, Shengzhong (Frank) Liu, Liming Ding. Defects in perovskite crystals[J]. Journal of Semiconductors, 2023, 44(8): 080201. doi: 10.1088/1674-4926/44/8/080201
****
Z M Fang, J Sun, S Z Liu, L M Ding. Defects in perovskite crystals[J]. J. Semicond, 2023, 44(8): 080201. doi: 10.1088/1674-4926/44/8/080201
|
-
References
[1] Zhang L X, Pan X Y, Liu L, et al. Star perovskite materials. J Semicond, 2022, 43, 030203 doi: 10.1088/1674-4926/43/3/030203[2] NREL, "Best Research-cell Efficiency Chart, " www. nrel. gov/pv/ cell- effic iency.html Accessed: Jun 2023[3] Zhou Y Y, Zhao Y X. Chemical stability and instability of inorganic halide perovskites. Energy Environ Sci, 2019, 12, 1495 doi: 10.1039/C8EE03559H[4] Yin W J, Shi T T, Yan Y F. Unique properties of halide perovskites as possible origins of the superior solar cell performance. Adv Mater, 2014, 26, 4653 doi: 10.1002/adma.201306281[5] Ran C X, Xu J T, Gao W Y, et al. Defects in metal triiodide perovskite materials towards high-performance solar cells: origin, impact, characterization, and engineering. Chem Soc Rev, 2018, 47, 4581 doi: 10.1039/C7CS00868F[6] Chen B, Rudd P N, Yang S, et al. Imperfections and their passivation in halide perovskite solar cells. Chem Soc Rev, 2019, 48, 3842 doi: 10.1039/C8CS00853A[7] Xu J X, Buin A, Ip A H, et al. Perovskite-fullerene hybrid materials suppress hysteresis in planar diodes. Nat Commun, 2015, 6, 7081 doi: 10.1038/ncomms8081[8] Shao Y C, Xiao Z G, Bi C, et al. Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells. Nat Commun, 2014, 5, 5784 doi: 10.1038/ncomms6784[9] Noel N K, Abate A, Stranks S D, et al. Enhanced photoluminescence and solar cell performance via lewis base passivation of organic-inorganic lead halide perovskites. ACS Nano, 2014, 8, 9815 doi: 10.1021/nn5036476[10] Wang F, Geng W, Zhou Y, et al. Phenylalkylamine passivation of organolead halide perovskites enabling high-efficiency and air-stable photovoltaic cells. Adv Mater, 2016, 28, 9986 doi: 10.1002/adma.201603062[11] Yang S, Dai J, Yu Z H, et al. Tailoring passivation molecular structures for extremely small open-circuit voltage loss in perovskite solar cells. J Am Chem Soc, 2019, 141, 5781 doi: 10.1021/jacs.8b13091[12] Li W Z, Dong H P, Guo X D, et al. Graphene oxide as dual functional interface modifier for improving wettability and retarding recombination in hybrid perovskite solar cells. J Mater Chem A, 2014, 2, 20105 doi: 10.1039/C4TA05196C[13] Cai Y, Cui J, Chen M, et al. Multifunctional enhancement for highly stable and efficient perovskite solar cells. Adv Funct Mater, 2021, 31, 2005776 doi: 10.1002/adfm.202005776[14] Lin Y Z, Shen L, Dai J, et al. π-conjugated lewis base: Efficient trap-passivation and charge-extraction for hybrid perovskite solar cells. Adv Mater, 2017, 29, 1604545 doi: 10.1002/adma.201604545[15] Jiang J X, Wang Q, Jin Z W, et al. Polymer doping for high-efficiency perovskite solar cells with improved moisture stability. Adv Energy Mater, 2018, 8, 1701757 doi: 10.1002/aenm.201701757[16] Zuo L J, Guo H X, deQuilettes D W, et al. Polymer-modified halide perovskite films for efficient and stable planar heterojunction solar cells. Sci Adv, 2017, 3, e1700106 doi: 10.1126/sciadv.1700106[17] Bi D Q, Yi C Y, Luo J S, et al. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat Energy, 2016, 1, 16142 doi: 10.1038/nenergy.2016.142[18] Li X, Dar M I, Yi C Y, et al. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkylphosphonic acid ω-ammonium chlorides. Nat Chem, 2015, 7, 703 doi: 10.1038/nchem.2324[19] Meng X Y, Lin J B, Liu X, et al. Highly stable and efficient FASnI3-based perovskite solar cells by introducing hydrogen bonding. Adv Mater, 2019, 31, 1903721 doi: 10.1002/adma.201903721[20] Wang R, Xue J J, Wang K L, et al. Constructive molecular configurations for surface-defect passivation of perovskite photovoltaics. Science, 2019, 366, 1509 doi: 10.1126/science.aay9698[21] Gong C, Zhang C, Zhuang Q X, et al. Stabilizing buried interface via synergistic effect of fluorine and sulfonyl functional groups toward efficient and stable perovskite solar cells. Nanomicro Lett, 2022, 15, 17 doi: 10.1007/s40820-022-00992-5[22] Abate A, Saliba M, Hollman D J, et al. Supramolecular halogen bond passivation of organic-inorganic halide perovskite solar cells. Nano Lett, 2014, 14, 3247 doi: 10.1021/nl500627x[23] Metrangolo P, Canil L, Abate A, et al. Halogen bonding in perovskite solar cells: A new tool for improving solar energy conversion. Angew Chem Int Ed, 2022, 61, e202114793 doi: 10.1002/anie.202114793[24] Ren G H, Han W B, Zhang Q, et al. Overcoming perovskite corrosion and de-doping through chemical binding of halogen bonds toward efficient and stable perovskite solar cells. Nano-Micro Lett, 2022, 14, 175 doi: 10.1007/s40820-022-00916-3[25] Zhang C Y, Shen X Q, Chen M J, et al. Constructing a stable and efficient buried heterojunction via halogen bonding for inverted perovskite solar cells. Adv Energy Mater, 2023, 13, 2203250 doi: 10.1002/aenm.202203250[26] Li N X, Tao S X, Chen Y H, et al. Cation and anion immobilization through chemical bonding enhancement with fluorides for stable halide perovskite solar cells. Nat Energy, 2019, 4, 408 doi: 10.1038/s41560-019-0382-6[27] Cao J, Tao S X, Bobbert P A, et al. Interstitial occupancy by extrinsic alkali cations in perovskites and its impact on ion migration. Adv Mater, 2018, 30, 1707350 doi: 10.1002/adma.201707350[28] Abdi-Jalebi M, Andaji-Garmaroudi Z, Cacovich S, et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature, 2018, 555, 497 doi: 10.1038/nature25989[29] Jiang Q, Zhao Y, Zhang X W, et al. Surface passivation of perovskite film for efficient solar cells. Nat Photon, 2019, 13, 460 doi: 10.1038/s41566-019-0398-2[30] Lin R X, Xu J, Wei M Y, et al. All-perovskite tandem solar cells with improved grain surface passivation. Nature, 2022, 603, 73 doi: 10.1038/s41586-021-04372-8[31] Zheng X P, Deng Y H, Chen B, et al. Dual functions of crystallization control and defect passivation enabled by sulfonic zwitterions for stable and efficient perovskite solar cells. Adv Mater, 2018, 30, 1803428 doi: 10.1002/adma.201803428[32] Zheng X P, Chen B, Dai J, et al. Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat Energy, 2017, 2, 17102 doi: 10.1038/nenergy.2017.102[33] Kim J H, Kim Y R, Park B, et al. Simultaneously passivating cation and anion defects in metal halide perovskite solar cells using a zwitterionic amino acid additive. Small, 2021, 17, 2005608 doi: 10.1002/smll.202005608[34] Chen Q, Zhou H P, Song T B, et al. Controllable self-induced passivation of hybrid lead iodide perovskites toward high performance solar cells. Nano Lett, 2014, 14, 4158 doi: 10.1021/nl501838y[35] Jacobsson T J, Correa-Baena J P, Anaraki E H, et al. Unreacted PbI2 as a double-edged sword for enhancing the performance of perovskite solar cells. J Am Chem Soc 2016, 138, 10331 doi: 10.1021/jacs.6b06320[36] Wang Z P, Lin Q Q, Chmiel F P, et al. Efficient ambient-air-stable solar cells with 2D–3D heterostructured butylammonium-caesium-formamidinium lead halide perovskites. Nat Energy, 2017, 2, 17135 doi: 10.1038/nenergy.2017.135[37] Yu D N, Wei Q, Li H S, et al. Quasi-2D bilayer surface passivation for high efficiency narrow bandgap perovskite solar cells. Angew Chem Int Ed, 2022, 61, e202202346 doi: 10.1002/anie.202202346[38] Leblebici S Y, Leppert L, Li Y B, et al. Facet-dependent photovoltaic efficiency variations in single grains of hybrid halide perovskite. Nat Energy, 2016, 1, 16093 doi: 10.1038/nenergy.2016.93[39] Fang Z M, Yan N, Liu S Z. Modulating preferred crystal orientation for efficient and stable perovskite solar cells—From progress to perspectives. InfoMat, 2022, 4, e12369 doi: 10.1002/inf2.12369[40] Zheng X P, Hou Y, Bao C X, et al. Managing grains and interfaces via ligand anchoring enables 22.3%-efficiency inverted perovskite solar cells. Nat Energy, 2020, 5, 131 doi: 10.1038/s41560-019-0538-4[41] Yan N, Ren X D, Fang Z M, et al. Ligand-anchoring-induced oriented crystal growth for high-efficiency lead-tin perovskite solar cells. Adv Funct Mater, 2022, 32, 2201384 doi: 10.1002/adfm.202201384[42] Li J G, Yan N, Fang Z M, et al. Alkyl diamine-induced (100)-preferred crystal orientation for efficient Pb–Sn perovskite solar cells. ACS Appl Energy Mater, 2022, 5, 6936 doi: 10.1021/acsaem.2c00587[43] Ma C Q, Eickemeyer F T, Lee S H, et al. Unveiling facet-dependent degradation and facet engineering for stable perovskite solar cells. Science, 2023, 379, 173 doi: 10.1126/science.adf3349[44] Ma C Q, Grätzel M, Park N G. Facet engineering for stable, efficient perovskite solar cells. ACS Energy Lett, 2022, 7, 3120 doi: 10.1021/acsenergylett.2c01623[45] Luo C, Zheng G H J, Gao F, et al. Facet orientation tailoring via 2D-seed-induced growth enables highly efficient and stable perovskite solar cells. Joule, 2022, 6, 240 doi: 10.1016/j.joule.2021.12.006 -
Proportional views





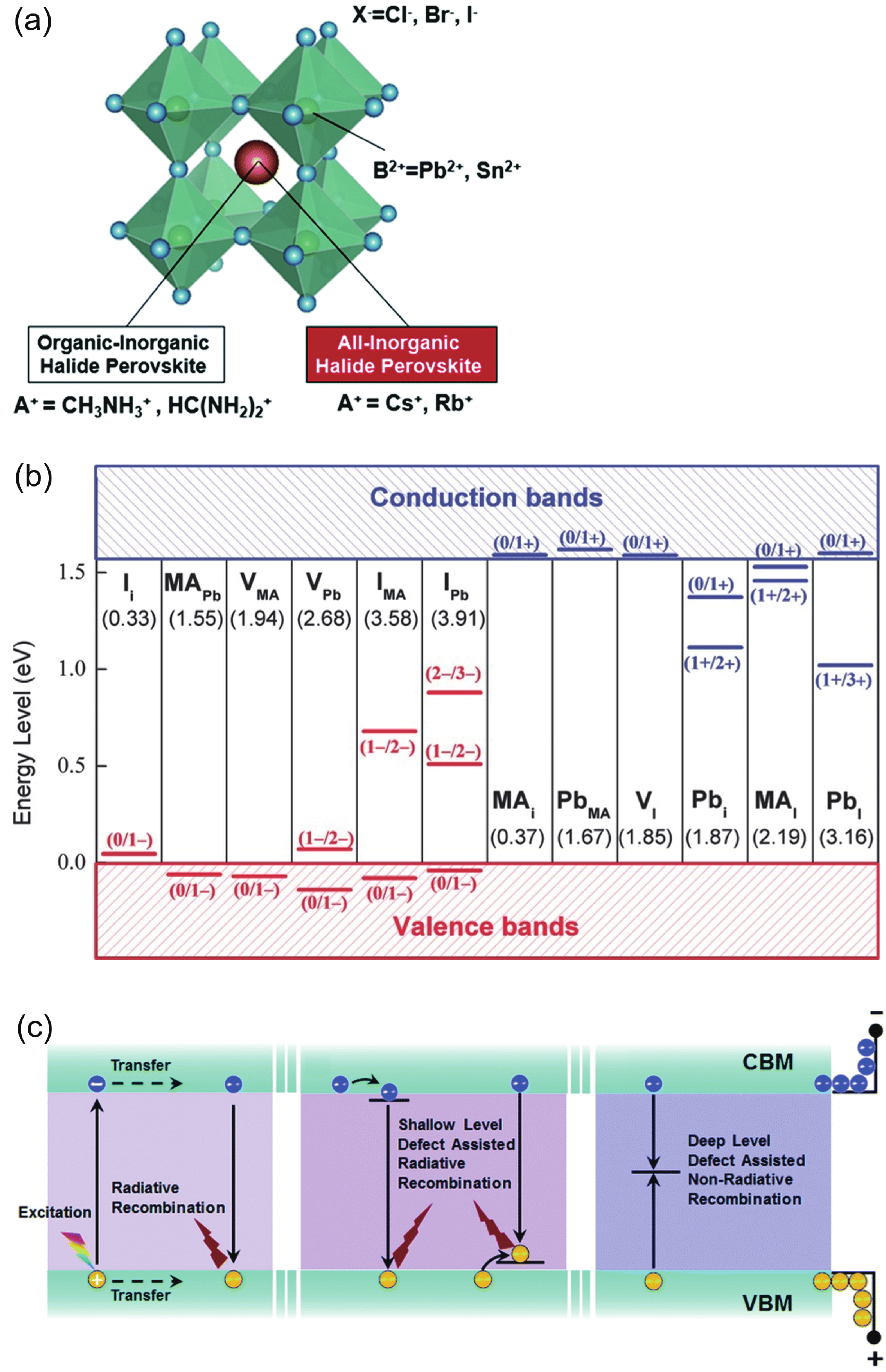
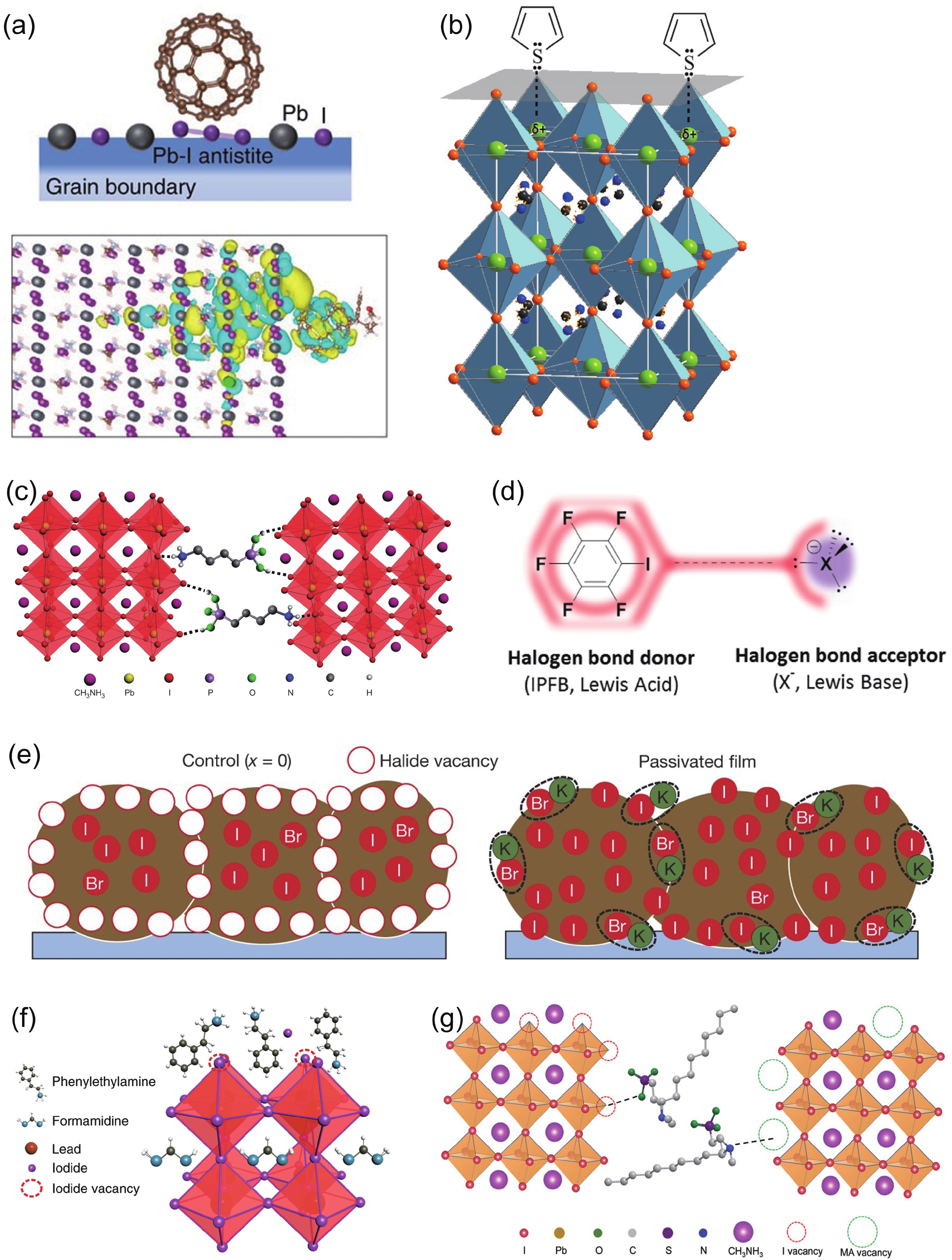
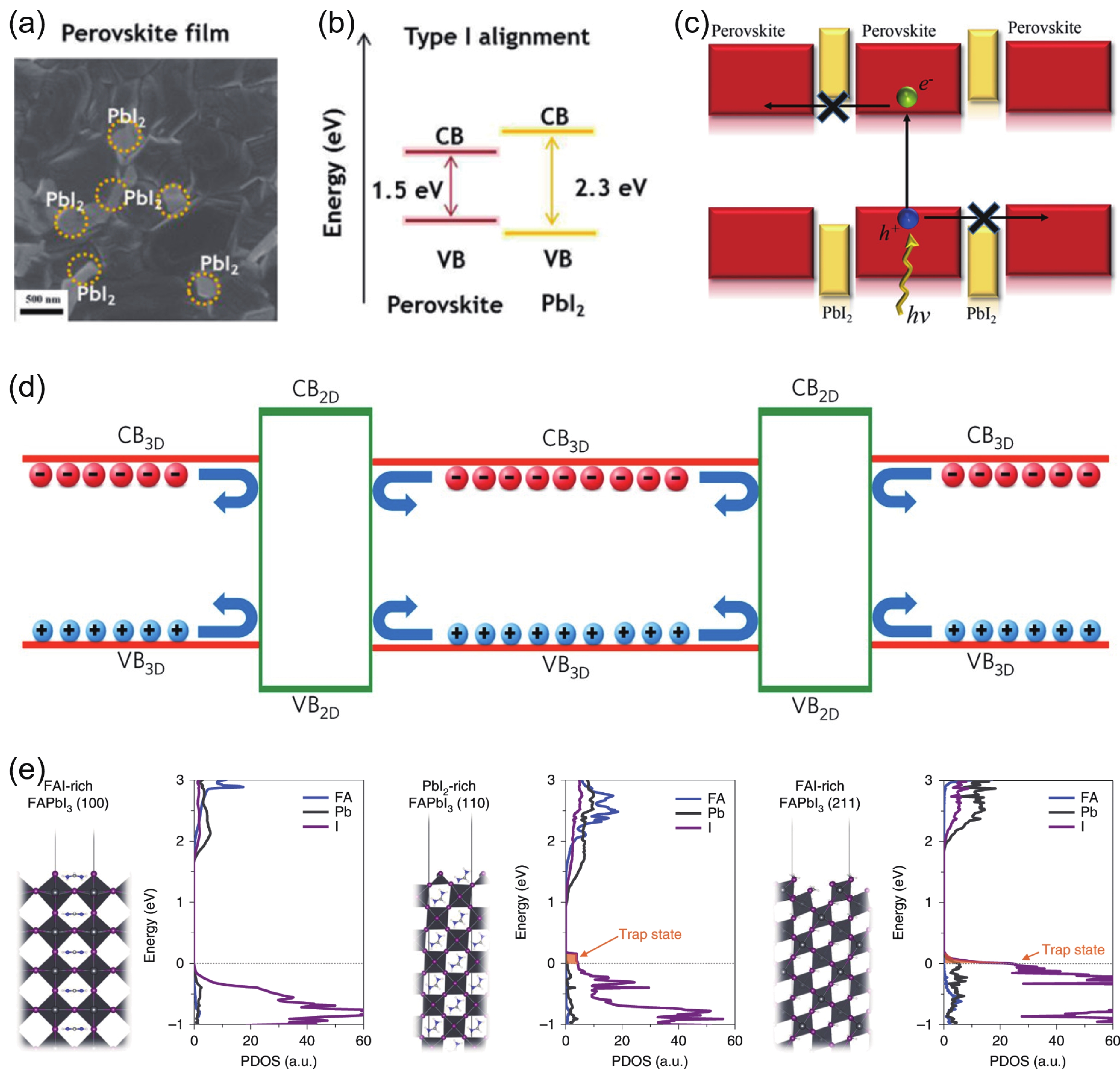
 DownLoad:
DownLoad:










 Zhimin Fang:got his PhD from University of Science and Technology of China in 2020. He started his research on perovskite solar cells under the supervision of Prof. Shangfeng Yang. Since September 2017, he worked in Liming Ding Laboratory at National Center for Nanoscience and Technology as a visiting student. In 2020, he joined Shengzhong Liu Group as a postdoc. His work focuses on perovskite-based tandem solar cells
Zhimin Fang:got his PhD from University of Science and Technology of China in 2020. He started his research on perovskite solar cells under the supervision of Prof. Shangfeng Yang. Since September 2017, he worked in Liming Ding Laboratory at National Center for Nanoscience and Technology as a visiting student. In 2020, he joined Shengzhong Liu Group as a postdoc. His work focuses on perovskite-based tandem solar cells Jie Sun:got her BS from Minzu University of China in 2021. Now she is a PhD student at University of Chinese Academy of Sciences under the supervision of Prof. Liming Ding. Her research focuses on perovskite devices
Jie Sun:got her BS from Minzu University of China in 2021. Now she is a PhD student at University of Chinese Academy of Sciences under the supervision of Prof. Liming Ding. Her research focuses on perovskite devices Shengzhong (Frank) Liu:received his PhD from Northwestern University in 1992. After his postdoctoral research at Argonne National Lab, he joined high-tech companies in US for research including nanoscale materials, thin-film solar cells, laser processing, diamond thin films, etc. His invention at BP Solar on semitransparent photovoltaic module won R & D 100 Awards in 2002. In 2011, he was selected into China talent program, and now he is a professor at Shaanxi Normal University
Shengzhong (Frank) Liu:received his PhD from Northwestern University in 1992. After his postdoctoral research at Argonne National Lab, he joined high-tech companies in US for research including nanoscale materials, thin-film solar cells, laser processing, diamond thin films, etc. His invention at BP Solar on semitransparent photovoltaic module won R & D 100 Awards in 2002. In 2011, he was selected into China talent program, and now he is a professor at Shaanxi Normal University Liming Ding:got his PhD from University of Science and Technology of China (was a joint student at Changchun Institute of Applied Chemistry, CAS). He started his research on OSCs and PLEDs in Olle Ingans Lab in 1998. Later on, he worked at National Center for Polymer Research, Wright-Patterson Air Force Base and Argonne National Lab (USA). He joined Konarka as a Senior Scientist in 2008. In 2010, he joined National Center for Nanoscience and Technology as a full professor. His research focuses on innovative materials and devices. He is RSC Fellow, and the Associate Editor for Journal of Semiconductors
Liming Ding:got his PhD from University of Science and Technology of China (was a joint student at Changchun Institute of Applied Chemistry, CAS). He started his research on OSCs and PLEDs in Olle Ingans Lab in 1998. Later on, he worked at National Center for Polymer Research, Wright-Patterson Air Force Base and Argonne National Lab (USA). He joined Konarka as a Senior Scientist in 2008. In 2010, he joined National Center for Nanoscience and Technology as a full professor. His research focuses on innovative materials and devices. He is RSC Fellow, and the Associate Editor for Journal of Semiconductors



