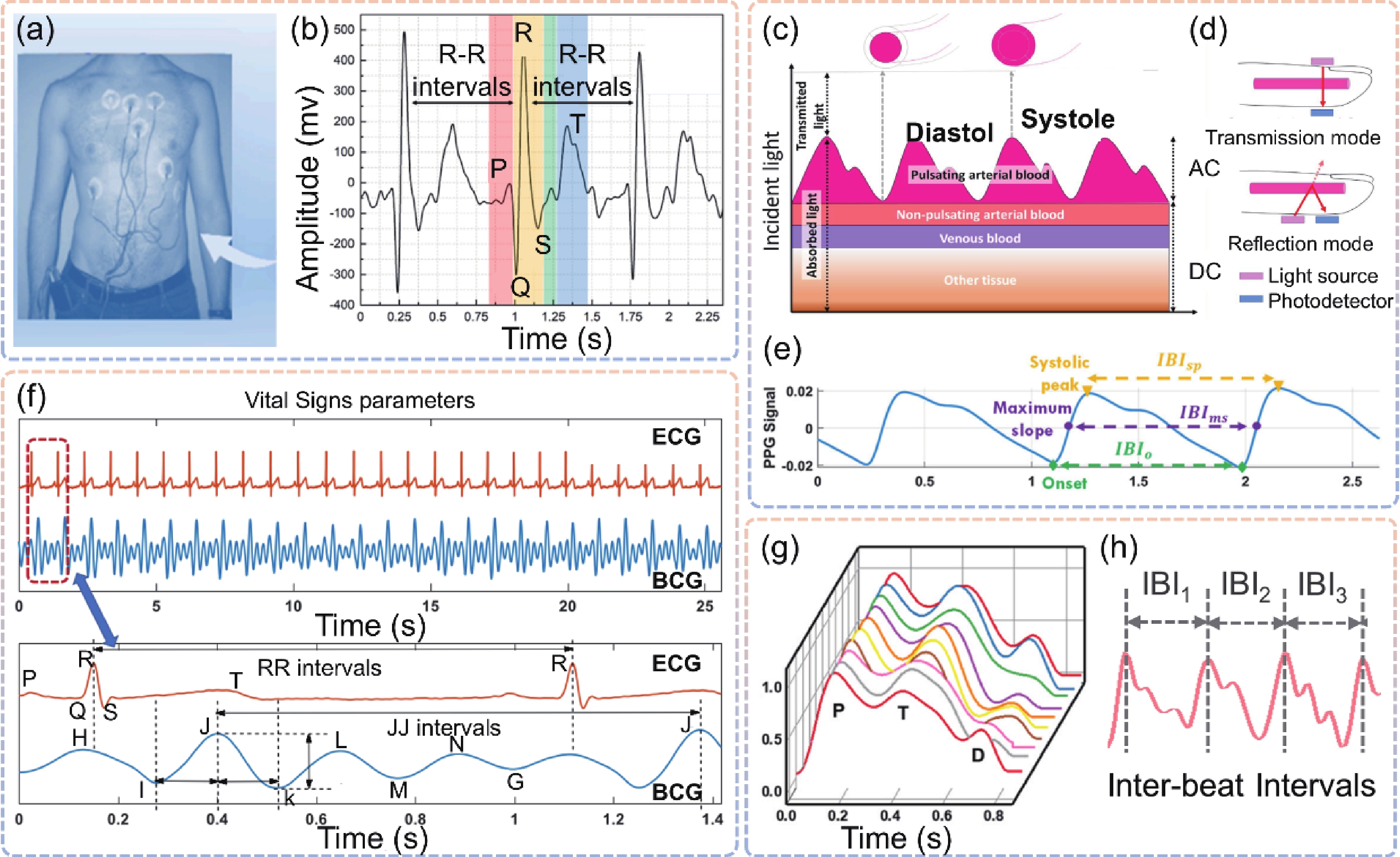
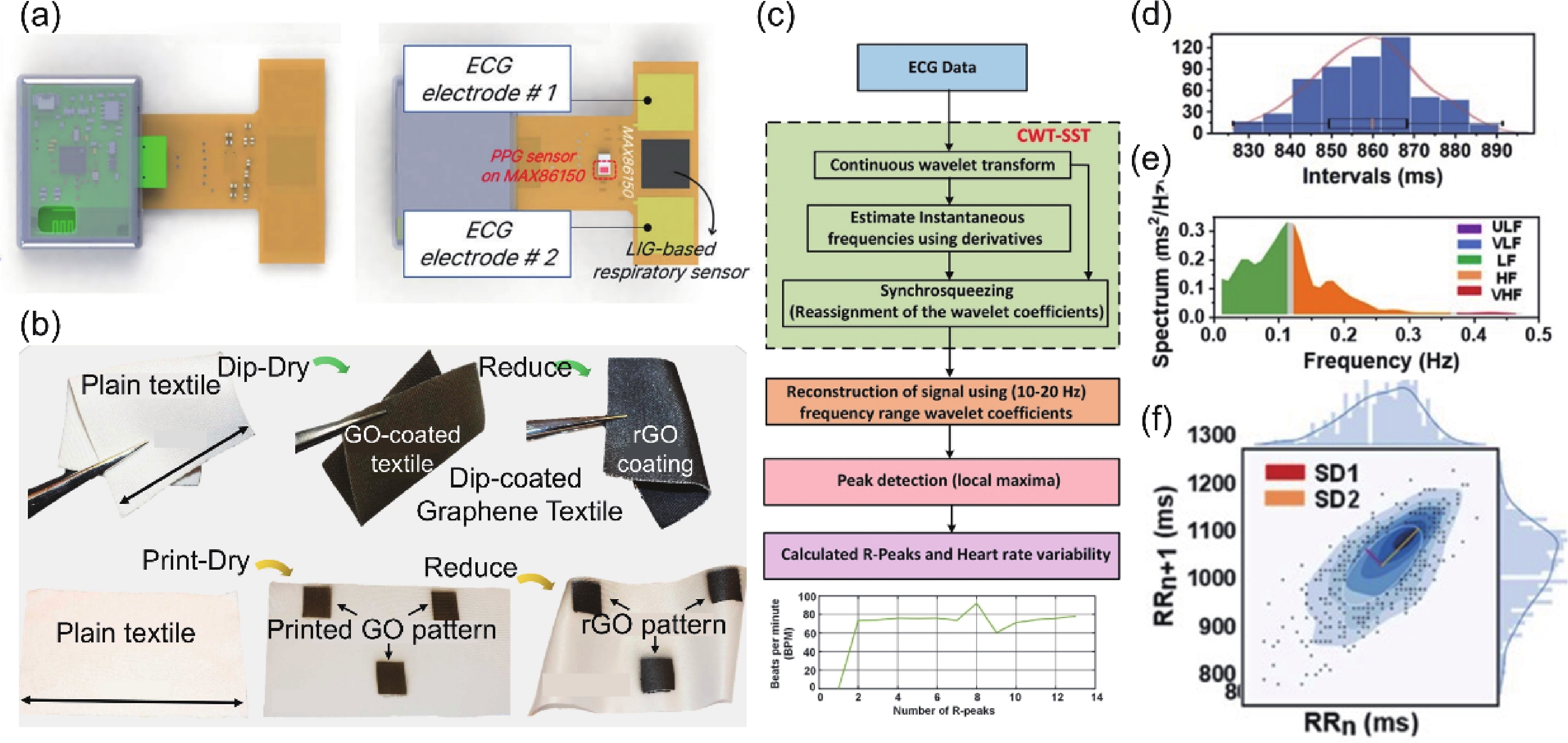
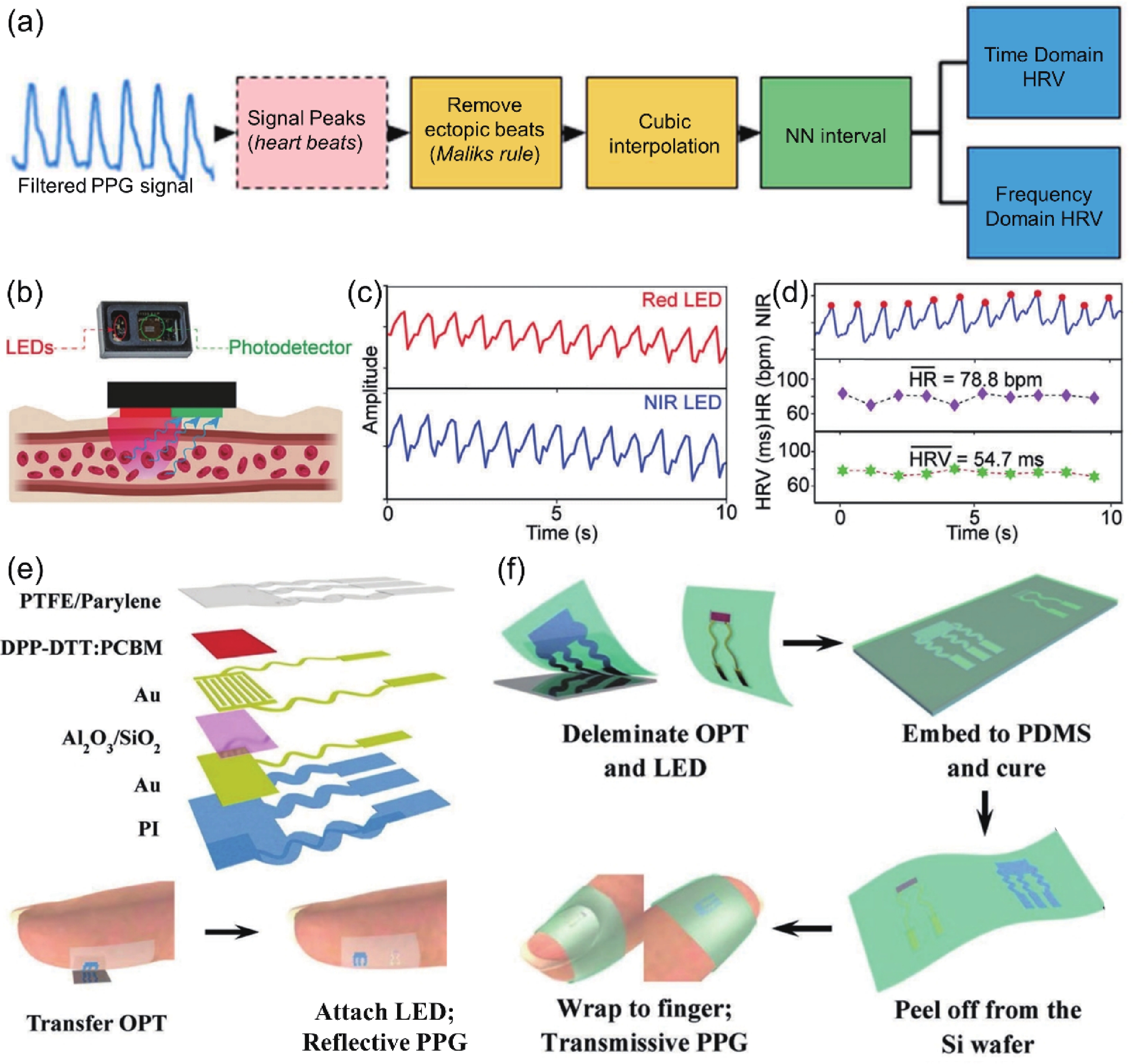
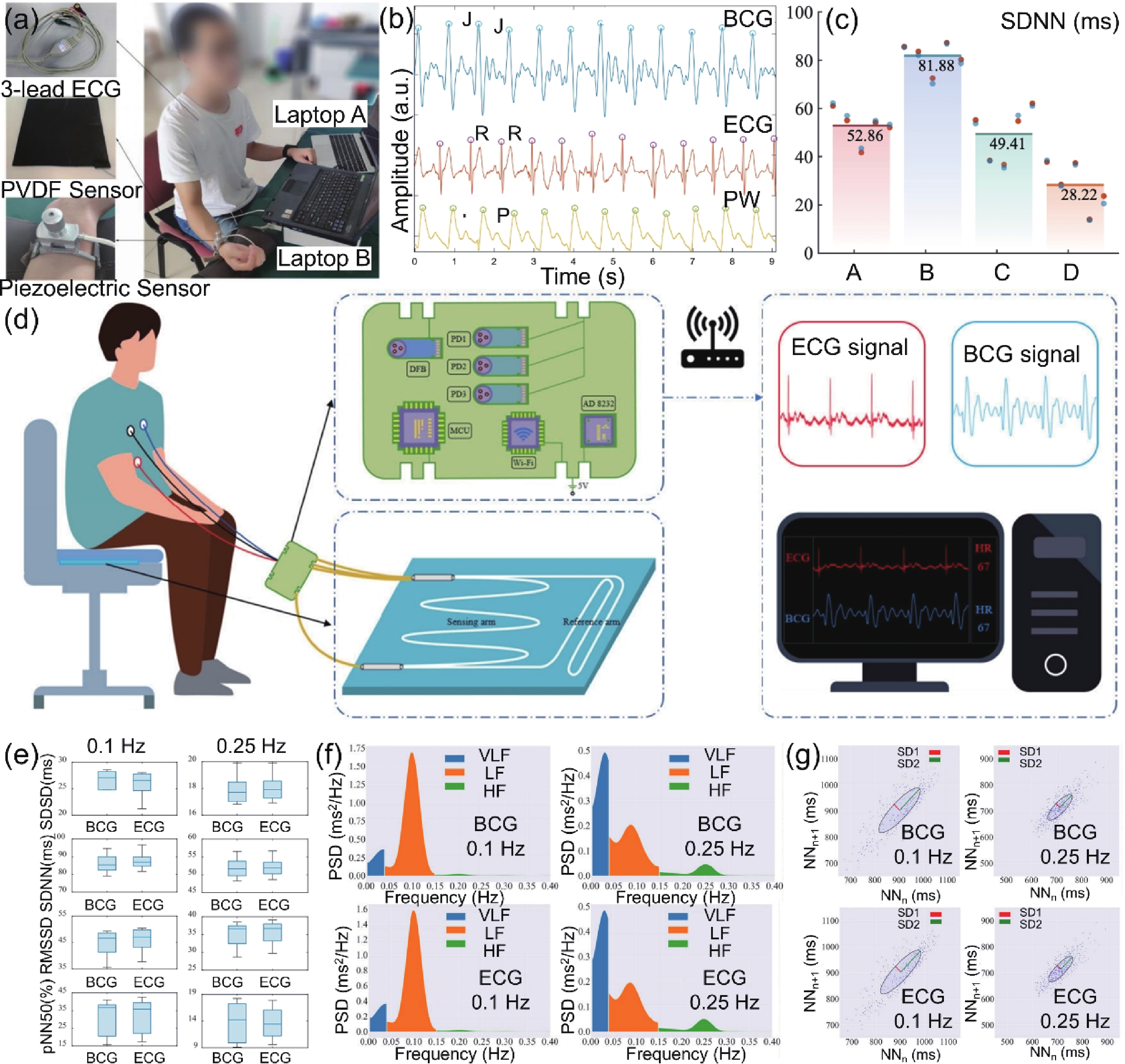
| Citation: |
Feifei Yin, Jian Chen, Haiying Xue, Kai Kang, Can Lu, Xinyi Chen, Yang Li. Integration of wearable electronics and heart rate variability for human physical and mental well-being assessment[J]. Journal of Semiconductors, 2025, 46(1): 011603. doi: 10.1088/1674-4926/24080026
****
F F Yin, J Chen, H Y Xue, K Kang, C Lu, X Y Chen, and Y Li, Integration of wearable electronics and heart rate variability for human physical and mental well-being assessment[J]. J. Semicond., 2025, 46(1), 011603 doi: 10.1088/1674-4926/24080026
|
Integration of wearable electronics and heart rate variability for human physical and mental well-being assessment
DOI: 10.1088/1674-4926/24080026
CSTR: 32376.14.1674-4926.24080026
More Information-
Abstract
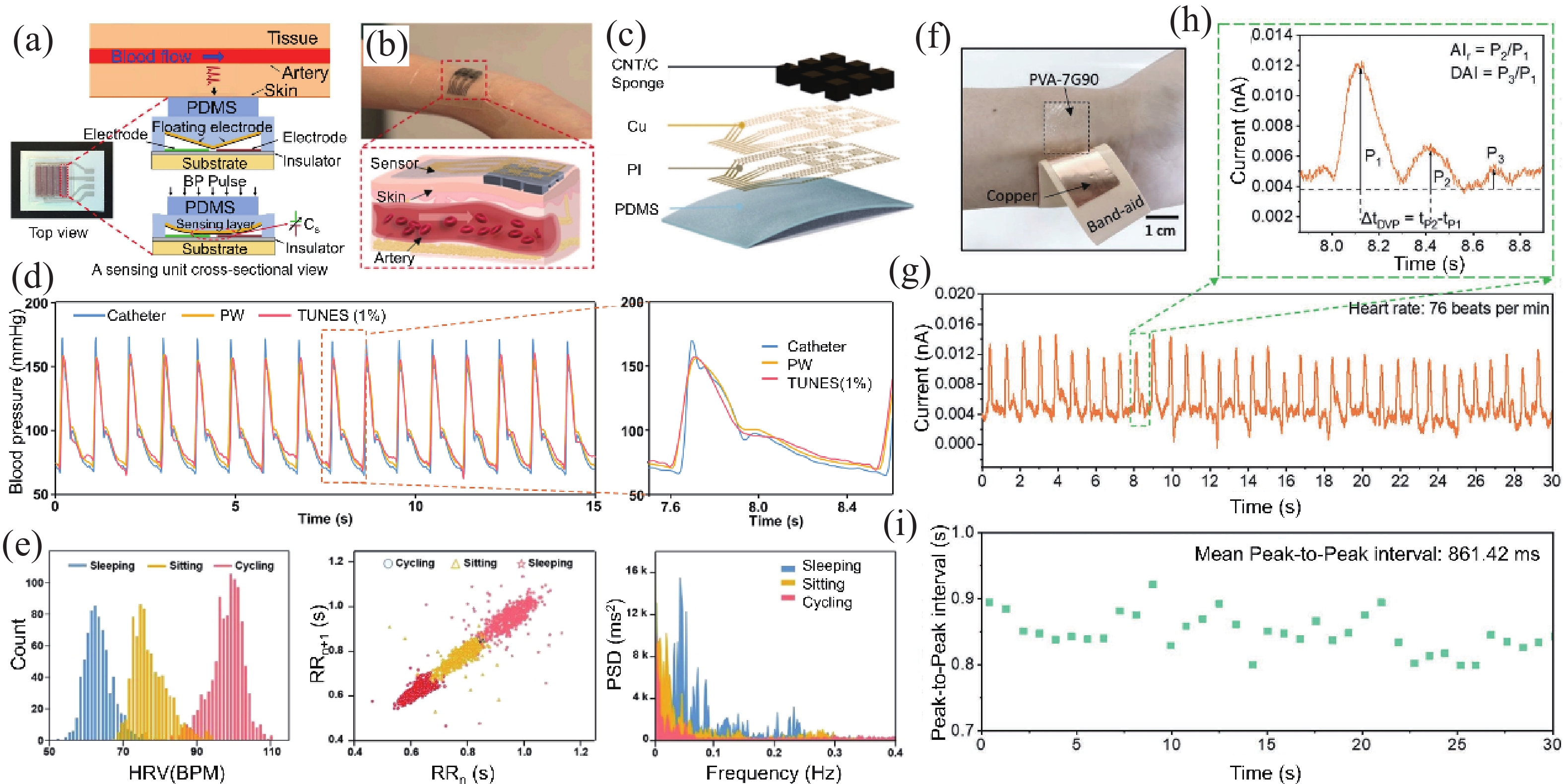
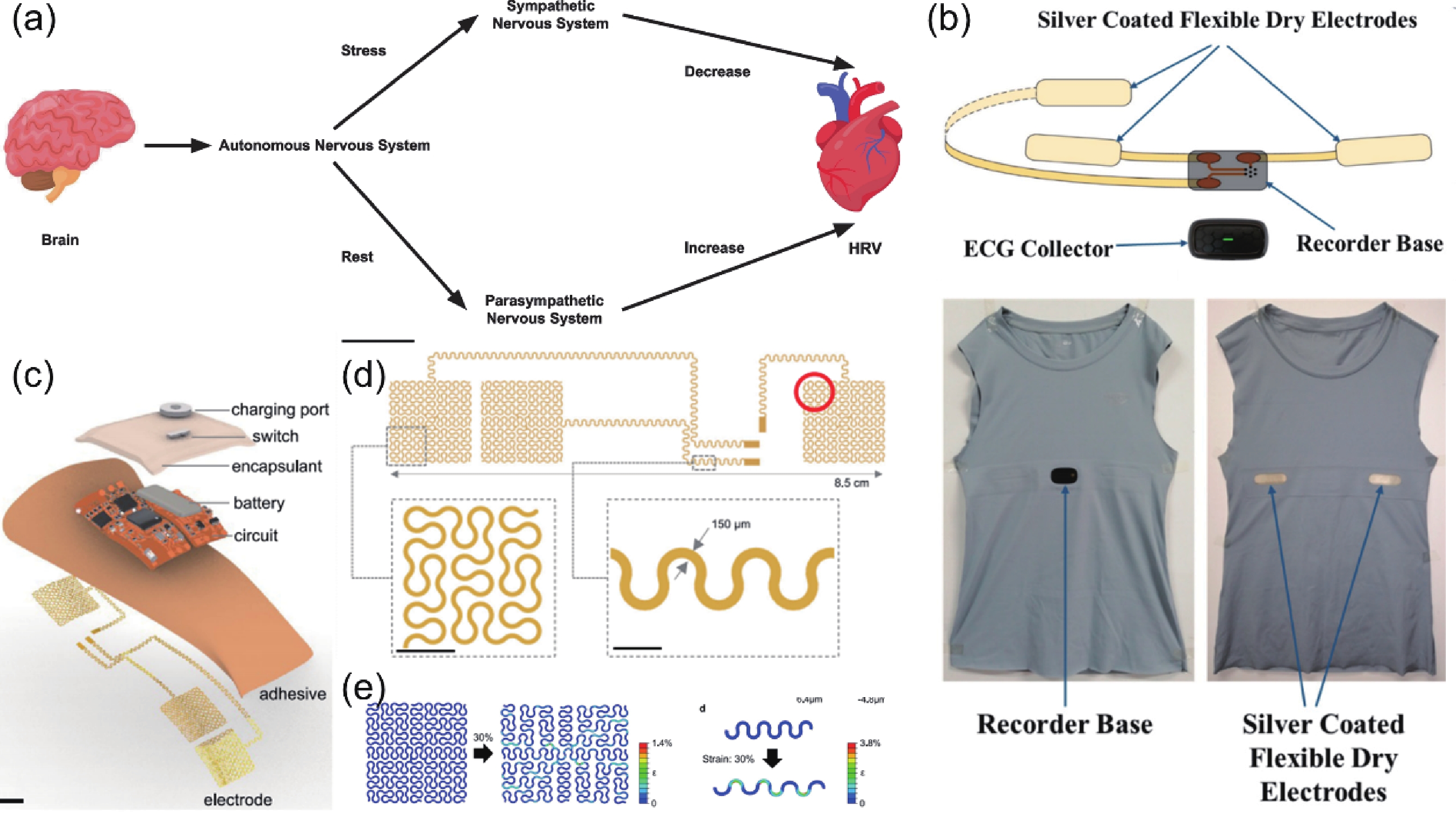
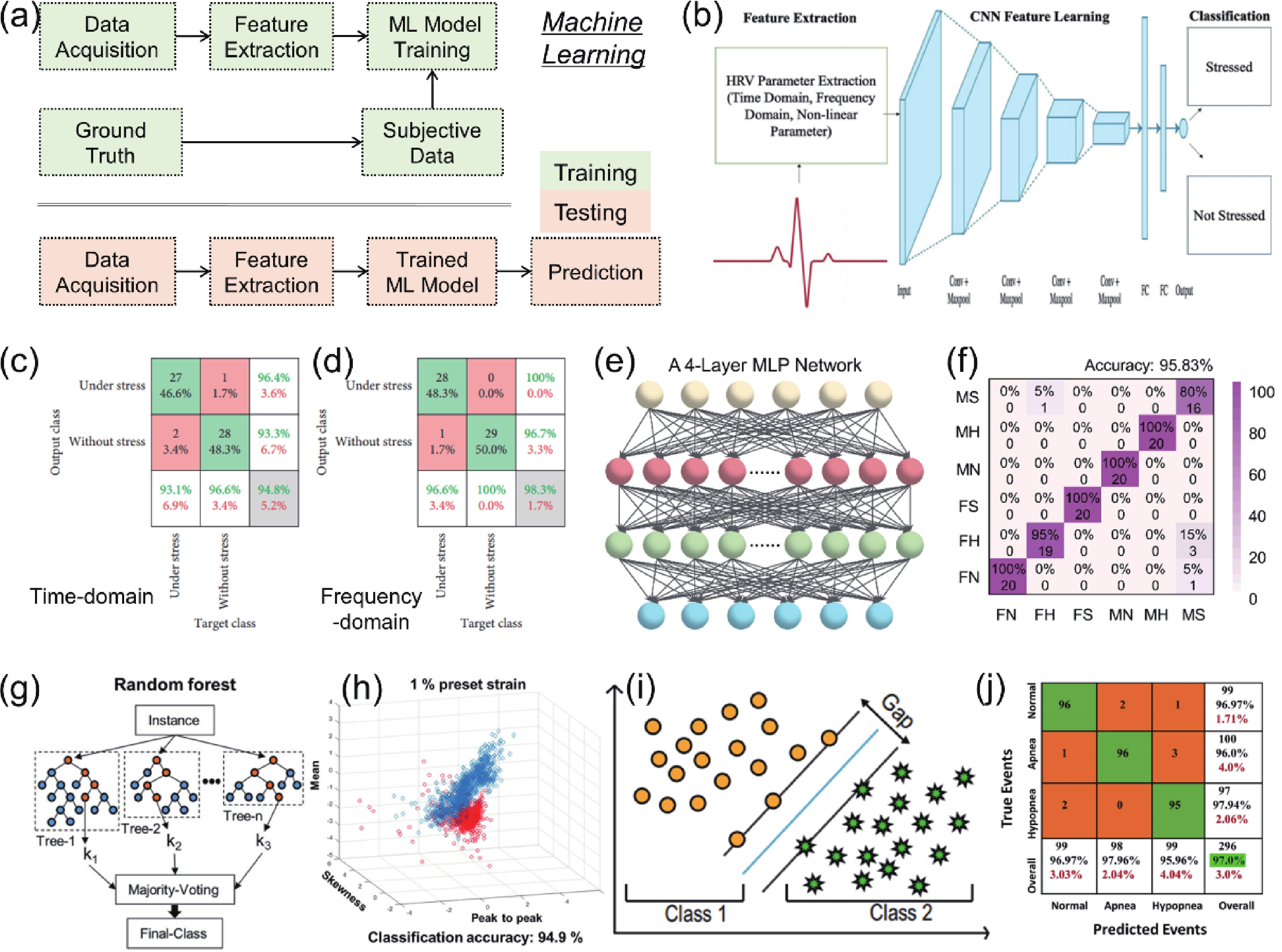
Heart rate variability (HRV) that can reflect the dynamic balance between the sympathetic nervous and parasympathetic nervous of human autonomic nervous system (ANS) has attracted considerable attention. However, traditional electrocardiogram (ECG) devices for HRV analysis are bulky, and hard wires are needed to attach measuring electrodes to the chest, resulting in the poor wearable experience during the long-term measurement. Compared with that, wearable electronics enabling continuously cardiac signals monitoring and HRV assessment provide a desirable and promising approach for helping subjects determine sleeping issues, cardiovascular diseases, or other threats to physical and mental well-being. Until now, significant progress and advances have been achieved in wearable electronics for HRV monitoring and applications for predicting human physical and mental well-being. In this review, the latest progress in the integration of wearable electronics and HRV analysis as well as practical applications in assessment of human physical and mental health are included. The commonly used methods and physiological signals for HRV analysis are briefly summarized. Furthermore, we highlighted the research on wearable electronics concerning HRV assessment and diverse applications such as stress estimation, drowsiness detection, etc. Lastly, the current limitations of the integrated wearable HRV system are concluded, and possible solutions in such a research direction are outlined. -
References
[1] Vanderlei L C M, Pastre C M, Hoshi R A, et al. Basic notions of heart rate variability and its clinical applicability. Braz J Cardiov Surg, 2009, 24, 205 doi: 10.1590/S0102-76382009000200018[2] Kreibig S D. Autonomic nervous system activity in emotion: A review. Biol Psychol, 2010, 84, 394 doi: 10.1016/j.biopsycho.2010.03.010[3] La Rovere M T, Gorini A, Schwartz P J. Stress, the autonomic nervous system, and sudden death. Auton Neurosci, 2022, 237, 102921 doi: 10.1016/j.autneu.2021.102921[4] Wessel J R, Danielmeier C, Ullsperger M. Error awareness revisited: accumulation of multimodal evidence from central and autonomic nervous systems. J Cogn Neurosci, 2011, 23, 3021 doi: 10.1162/jocn.2011.21635[5] Laborde S, Mosley E, Bellenger C, et al. Editorial: Horizon 2030: Innovative applications of heart rate variability. Front Neurosci, 2022, 16, 937086 doi: 10.3389/fnins.2022.937086[6] McCraty R, Shaffer F. Heart rate variability: new perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob Adv Health Med, 2015, 4, 46 doi: 10.7453/gahmj.2014.073[7] Gullett N, Zajkowska Z, Walsh A, et al. Heart rate variability (HRV) as a way to understand associations between the autonomic nervous system (ANS) and affective states: A critical review of the literature. International Journal of Psychophysiology, 2023, 192, 35 doi: 10.1016/j.ijpsycho.2023.08.001[8] Rajendra Acharya U, Paul Joseph K, Kannathal N, et al. Heart rate variability: a review. Med Biol Eng Comput, 2006, 44, 1031 doi: 10.1007/s11517-006-0119-0[9] Ranpuria R, Hall M, Chan C T, et al. Heart rate variability (HRV) in kidney failure: measurement and consequences of reduced HRV. Nephrol Dial Transplant, 2008, 23, 444 doi: 10.1093/ndt/gfm634[10] Stein P K, Pu Y C. Heart rate variability, sleep and sleep disorders. Sleep Med Rev, 2012, 16, 47 doi: 10.1016/j.smrv.2011.02.005[11] Thayer J F, Ahs F, Fredrikson M, et al. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev, 2012, 36, 747 doi: 10.1016/j.neubiorev.2011.11.009[12] Schwerdtfeger A R, Schwarz G, Pfurtscheller K, et al. Heart rate variability (HRV): From brain death to resonance breathing at 6 breaths per minute. Clin Neurophysiol, 2020, 131, 676 doi: 10.1016/j.clinph.2019.11.013[13] Castaldo R, Melillo P, Bracale U, et al. Acute mental stress assessment via short term HRV analysis in healthy adults: A systematic review with meta-analysis. Biomed Signal Process Contr, 2015, 18, 370 doi: 10.1016/j.bspc.2015.02.012[14] Beauchaine T P, Thayer J F. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int J Psychophysiol, 2015, 98, 338 doi: 10.1016/j.ijpsycho.2015.08.004[15] Billman G E. Heart rate variability-a historical perspective. Front Physiol, 2011, 2, 86 doi: 10.3389/fphys.2011.00086[16] Aubert A E, Seps B, Beckers F. Heart rate variability in athletes. Sports Med, 2003, 33, 889 doi: 10.2165/00007256-200333120-00003[17] Berntson G G, Thomas Bigger JR J, Eckberg D L, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology, 1997, 34, 623 doi: 10.1111/j.1469-8986.1997.tb02140.x[18] Owens A P. The role of heart rate variability in the future of remote digital biomarkers. Front Neurosci, 2020, 14, 582145 doi: 10.3389/fnins.2020.582145[19] Khunti K. Accurate interpretation of the 12-lead ECG electrode placement: A systematic review. Health Education Journal, 2014, 73, 610 doi: 10.1177/0017896912472328[20] Reyna M A, Alday E A P, Gu A N, et al. Classification of 12-lead ECGs: The PhysioNet/computing in cardiology challenge 2020. 2020 Computing in Cardiology, 2020, 1 doi: 10.22489/CinC.2020.236[21] Xiong P, Lee S M Y, Chan G. Deep learning for detecting and locating myocardial infarction by electrocardiogram: A literature review. Front Cardiovasc Med, 2022, 9, 860032 doi: 10.3389/fcvm.2022.860032[22] Ma C B, Lan K, Wang J, et al. Arrhythmia detection based on multi-scale fusion of hybrid deep models from single lead ECG recordings: A multicenter dataset study. Biomed. Biomed Signal Process Contr, 2022, 77, 103753 doi: 10.1016/j.bspc.2022.103753[23] Ribeiro A H, Ribeiro M H, Paixão G M M, et al. Automatic diagnosis of the 12-lead ECG using a deep neural network. Nat Commun, 2020, 11, 1760 doi: 10.1038/s41467-020-15432-4[24] Yoo J, Yan L, Lee S, et al. A wearable ECG acquisition system with compact planar-fashionable circuit board-based shirt. IEEE Trans Inf Technol Biomed, 2009, 13, 897 doi: 10.1109/TITB.2009.2033053[25] Bolourchi M, Silver E S, Muwanga D, et al. Comparison of holter with Zio patch electrocardiography monitoring in children. Am J Cardiol, 2020, 125, 767 doi: 10.1016/j.amjcard.2019.11.028[26] Zhao S F, Ran W H, Wang L L, et al. Interlocked MXene/rGO aerogel with excellent mechanical stability for a health-monitoring device. J Semicond, 2022, 43, 082601 doi: 10.1088/1674-4926/43/8/082601[27] Hua Q L, Shen G Z. A wearable sweat patch for non-invasive and wireless monitoring inflammatory status. J Semicond, 2023, 44, 100401 doi: 10.1088/1674-4926/44/10/100401[28] Niu H, Li H, Li N, et al. Fringing-effect-based capacitive proximity sensors. Adv Funct Mater, 2024, 2409820. doi: 10.1002/adfm.202409820[29] Zhang H Y, Li H, Li Y, et al. Biomimetic electronic skin for robots aiming at superior dynamic-static perception and material cognition based on triboelectric-piezoresistive effects. Nano Lett, 2024, 24, 4002 doi: 10.1021/acs.nanolett.4c00623[30] Jerath R, Syam M, Ahmed S. The future of stress management: integration of smartwatches and HRV technology. Sensors, 2023, 23, 7314 doi: 10.3390/s23177314[31] Jiang W, Majumder S, Kumar S, et al. A wearable tele-health system towards monitoring COVID-19 and chronic diseases. IEEE Rev Biomed Eng, 2021, 15, 61 doi: 10.1109/RBME.2021.3069815[32] Qiu S, Zhao H, Jiang N, et al. Multi-sensor information fusion based on machine learning for real applications in human activity recognition: State-of-the-art and research challenges. Inf Fusion, 2022, 80, 241 doi: 10.1016/j.inffus.2021.11.006[33] Mishra S, Mohanty S, Ramadoss A. Functionality of flexible pressure sensors in cardiovascular health monitoring: A review. ACS Sens, 2022, 7, 2495 doi: 10.1021/acssensors.2c00942[34] Lin J, Fu R, Zhong X, et al. Wearable sensors and devices for real-time cardiovascular disease monitoring. Cell Rep Phys Sci, 2021, 2, 100541 doi: 10.1016/j.xcrp.2021.100541[35] Kwon S H, Dong L. Flexible sensors and machine learning for heart monitoring. Nano Energy, 2022, 102, 107632 doi: 10.1016/j.nanoen.2022.107632[36] Taoum A, Bisiaux A, Tilquin F, et al. Validity of ultra-short-term HRV analysis using PPG-a preliminary study. Sensors, 2022, 22, 7995 doi: 10.3390/s22207995[37] Shaffer F, Ginsberg J P. An overview of heart rate variability metrics and norms. Front Public Health, 2017, 5, 258 doi: 10.3389/fpubh.2017.00258[38] Kuusela T. Methodological aspects of heart rate variability analysis. Heart rate variability (HRV) signal analysis: CRC Press, 2012, 9[39] Malik M, Bigger J T, Camm A J, et al. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur Heart J, 1996, 17, 354 doi: 10.1093/oxfordjournals.eurheartj.a014868[40] Grant C C, van Rensburg D C, Strydom N, et al. Importance of tachogram length and period of recording during noninvasive investigation of the autonomic nervous system. Ann Noninvasive Electrocardiol, 2011, 16, 131 doi: 10.1111/j.1542-474X.2011.00422.x[41] Laborde S, Mosley E, Thayer J F. Heart rate variability and cardiac vagal tone in psychophysiological research-recommendations for experiment planning, data analysis, and data reporting. Front Psychol, 2017, 8, 213 doi: 10.3389/fpsyg.2017.00213[42] Gouveia C, Albuquerque D F, Pinho P, et al. Bio-radar cardiac signal model used for HRV assessment and evaluation using adaptive filtering. IEEE Trans Instrum Meas, 2022, 71, 8503810 doi: 10.1109/TIM.2022.3190035[43] Kleiger R E, Miller J P, Bigger J T Jr, et al. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol, 1987, 59, 256 doi: 10.1016/0002-9149(87)90795-8[44] Shaffer F, McCraty R, Zerr C L. A healthy heart is not a metronome: an integrative review of the hearts anatomy and heart rate variability. Front Psychol, 2014, 5, 1040 doi: 10.3389/fpsyg.2014.01040[45] Hill L K, Siebenbrock A, Sollers J J, et al. Are all measures created equal? Heart rate variability and respiration. Biomed Sci Instrum, 2009, 45, 71[46] DeGiorgio C M, Miller P, Meymandi S, et al. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav, 2010, 19, 78 doi: 10.1016/j.yebeh.2010.06.011[47] Tsuji H, Venditti F J Jr, Manders E S, et al. Reduced heart rate variability and mortality risk in an elderly cohort. The Framingham Heart Study. Circulation, 1994, 90, 878 doi: 10.1161/01.CIR.90.2.878[48] Tsuji H, Larson M G, Venditti F J Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation, 1996, 94, 2850 doi: 10.1161/01.CIR.94.11.2850[49] Hadase M, Azuma A, Zen K, et al. Very low frequency power of heart rate variability is a powerful predictor of clinical prognosis in patients with congestive heart failure. Circ J, 2004, 68, 343 doi: 10.1253/circj.68.343[50] Quintana D S, Elstad M, Kaufmann T, et al. Resting-state high-frequency heart rate variability is related to respiratory frequency in individuals with severe mental illness but not healthy controls. Sci Rep, 2016, 6, 37212 doi: 10.1038/srep37212[51] Grossman P, Taylor E W. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol, 2007, 74, 263 doi: 10.1016/j.biopsycho.2005.11.014[52] Kleiger R E, Stein P K, Bigger J T Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol, 2005, 10, 88 doi: 10.1111/j.1542-474X.2005.10101.x[53] Carney R M, Freedland K E, Stein P K, et al. Heart rate variability and markers of inflammation and coagulation in depressed patients with coronary heart disease. J Psychosom Res, 2007, 62, 463 doi: 10.1016/j.jpsychores.2006.12.004[54] Francis J. ECG monitoring leads and special leads. Indian Pacing Electrophysiol J, 2016, 16, 92 doi: 10.1016/j.ipej.2016.07.003[55] Agostinelli A, Giuliani C, Burattini L. Extracting a clean ECG from a noisy recording: a new method based on segmented-beat modulation. Computing in Cardiology, 2014, 49[56] Ryu G S, You J, Kostianovskii V, et al. Flexible and printed PPG sensors for estimation of drowsiness. IEEE Trans Electron Devices, 2018, 65, 2997 doi: 10.1109/TED.2018.2833477[57] Alvarado-Serrano C, Luna-Lozano P S, Pallas-Areny R. An algorithm for beat-to-beat heart rate detection from the BCG based on the continuous spline wavelet transform. Biomedical Signal Processing and Control, 2016, 27, 96 doi: 10.1016/j.bspc.2016.02.002[58] Garcia-Limon J A, Flores-Nunez L I, Alvarado-Serrano C, et al. Novel algorithm for beat-to-beat heart rate measurement from the BCG in seated, standing and supine positions: Towards an universal algorithm. Biomedical Signal Process, 2024, 96, 106641 doi: 10.1016/j.bspc.2024.106641[59] Lim H R, Kim H S, Qazi R, et al. Advanced soft materials, sensor integrations, and applications of wearable flexible hybrid electronics in healthcare, energy, and environment. Adv Mater, 2020, 32, 1901924 doi: 10.1002/adma.201901924[60] Lee E K, Kim M K, Lee C H. Skin-mountable biosensors and therapeutics: a review. Annu Rev Biomed Eng, 2019, 21, 299 doi: 10.1146/annurev-bioeng-060418-052315[61] Yang Y N, Shi L J, Cao Z R, et al. Strain sensors with a high sensitivity and a wide sensing range based on a Ti3C2Tx(MXene) nanoparticle–nanosheet hybrid network. Adv Funct Mater, 2019, 29, 1807882 doi: 10.1002/adfm.201807882[62] Cui H, Wang Z, Yu B, et al. Statistical analysis of the consistency of HRV analysis using bcg or pulse wave signals. Sensors, 2022, 22, 2423 doi: 10.3390/s22062423[63] Kim T, Hong I, Roh Y, et al. Spider-inspired tunable mechanosensor for biomedical applications. NPJ Flex Electron, 2023, 7, 12 doi: 10.1038/s41528-023-00247-2[64] Fang P, Peng Y, Lin W H, et al. Wrist pulse recording with a wearable piezoresistor-piezoelectret compound sensing system and its applications in health monitoring. IEEE Sens J, 2021, 21, 20921 doi: 10.1109/JSEN.2021.3094845[65] Huang Z, Gao M, Yan Z, et al. Pyramid microstructure with single walled carbon nanotubes for flexible and transparent micro-pressure sensor with ultra-high sensitivity. Sens Actuat A Phys, 2017, 266, 345 doi: 10.1016/j.sna.2017.09.054[66] Yuan S, Bai J, Li S Z, et al. A multifunctional and selective ionic flexible sensor with high environmental suitability for tactile perception. Adv Funct Mater, 2024, 34, 2309626 doi: 10.1002/adfm.202309626[67] Yan Z C, Pan T S, Wang D K, et al. Stretchable micromotion sensor with enhanced sensitivity using serpentine layout. ACS Appl Mater Interfaces, 2019, 11, 12261 doi: 10.1021/acsami.8b22613[68] Wang X, Feng Z P, Xia Y S, et al. Flexible pressure sensor for high-precision measurement of epidermal arterial pulse. Nano Energy, 2022, 102, 107710 doi: 10.1016/j.nanoen.2022.107710[69] Niu H S, Gao S, Yue W J, et al. Highly morphology-controllable and highly sensitive capacitive tactile sensor based on epidermis-dermis-inspired interlocked asymmetric-nanocone arrays for detection of tiny pressure. Small, 2020, 16, 1904774 doi: 10.1002/smll.201904774[70] Yin F F, Niu H S, Shin Y K, et al. A wearable device based on the ionic liquid decorated sponge-like ultraviolet-curable resin for recognizing human mental health conditions. Nano Energy, 2023, 118, 109039 doi: 10.1016/j.nanoen.2023.109039[71] McAdams E, Krupaviciute A, Gehin C, et al. Wearable electronic systems: Applications to medical diagnostics/monitoring. In Wearable monitoring systems, 2010, 179[72] Ozturk O, Golparvar A, Acar G, et al. Single-arm diagnostic electrocardiography with printed graphene on wearable textiles. Sens Actuat A Phys, 2023, 349, 114058 doi: 10.1016/j.sna.2022.114058[73] Aygun A, Ghasemzadeh H, Jafari R. Robust interbeat interval and heart rate variability estimation method from various morphological features using wearable sensors. IEEE J Biomed Health Inform, 2019, 24, 2238 doi: 10.1109/JBHI.2019.2962627[74] Jia H, Gao Y, Zhou J, et al. A deep learning-assisted skin-integrated pulse sensing system for reliable pulse monitoring and cardiac function assessment. Nano Energy, 2024, 109796. doi: 10.1016/j.nanoen.2024.109796[75] Yokota Y, Aoki M, Mizuta K, et al. Motion sickness susceptibility associated with visually induced postural instability and cardiac autonomic responses in healthy subjects. Acta Oto Laryngol, 2005, 125, 280 doi: 10.1080/00016480510003192[76] Doweck I, Gordon C R, Shlitner A, et al. Alterations in r-r variability associated with experimental motion sickness. J Auton Nerv Syst, 1997, 67, 31 doi: 10.1016/S0165-1838(97)00090-8[77] Benedek M, Kaernbach C. A continuous measure of phasic electrodermal activity. J Neurosci Methods, 2010, 190, 80 doi: 10.1016/j.jneumeth.2010.04.028[78] Chawla N V, Bowyer K W, Hall L O, et al. SMOTE: Synthetic minority over-sampling technique. Jair, 2002, 16, 321 doi: 10.1613/jair.953[79] Bin Heyat M B, Akhtar F, Abbas S J, et al. Wearable flexible electronics based cardiac electrode for researcher mental stress detection system using machine learning models on single lead electrocardiogram signal. Biosensors, 2022, 12, 427 doi: 10.3390/bios12060427[80] Lee J N, Rachim V P, Hwang Y M, et al. Modular-Hybrid wearable cardiopulmonary monitoring sensor for unobstructive critical care: with a demonstration in practice. IEEE Sens J, 2024, 24, 8763 doi: 10.1109/JSEN.2024.3359215[81] Sukumar N, Sivashankar B, Sumathi P, et al. Physiological and physical sensors for stress level, drowsiness detection, and behaviour analysis. IEEE Trans Consum Electron, 2024, 70, 656 doi: 10.1109/TCE.2024.3366988[82] Yan W, Ma C, Cai X, et al. Self-powered and wireless physiological monitoring system with integrated power supply and sensors. Nano Energy, 2023, 108, 108203 doi: 10.1016/j.nanoen.2023.108203[83] Pham T, Lau Z J, Chen S H A, et al. Heart rate variability in psychology: A review of HRV indices and an analysis tutorial. Sensors, 2021, 21, 3998 doi: 10.3390/s21123998[84] Peng S, Xu K, Bao S J, et al. Flexible electrodes-based smart mattress for monitoring physiological signals of heart and autonomic nerves in a non-contact way. IEEE Sens J, 2020, 21, 6 doi: 10.1109/JSEN.2020.3012697[85] Gao Y, Soman V V, Lombardi J P, et al. Heart monitor using flexible capacitive ECG electrodes. IEEE Trans Instrum Meas, 2019, 69, 4314 doi: 10.1109/TIM.2019.2949320[86] Stephenson A C, Willis R, Alford C. Using in-seat electrical potential sensors for non-contact monitoring of heart rate, heart rate variability, and heart rate recovery. Int J Psychophysiol, 2021, 169, 1 doi: 10.1016/j.ijpsycho.2021.08.005[87] Huang J J, Cai Z L. Using flexible curved noncontact active electrodes to monitor long-term heart rate variability. J Healthc Eng, 2020, 2020, 8867712 doi: 10.1155/2020/8867712[88] Schwartz G, Tee B C K, Mei J G, et al. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat Commun, 2013, 4, 1859 doi: 10.1038/ncomms2832[89] Zhao B B, Wang Z, Yu Z W, et al. EmotionSense: emotion recognition based on wearable wristband. 2018 IEEE SmartWorld, Ubiquitous Intelligence & Computing, Advanced & Trusted Computing, Scalable Computing & Communications, Cloud & Big Data Computing, Internet of People and Smart City Innovation (SmartWorld/SCALCOM/UIC/ATC/CBDCom/IOP/SCI), 2018, 346 doi: 10.1109/smartworld.2018.00091[90] Singh J, Ameenpur S, Ahmed R, et al. An observational study of heart rate variability using wearable sensors provides a target for therapeutic monitoring of autonomic dysregulation in patients with Rett syndrome. Biomedicines, 2022, 10, 1684 doi: 10.3390/biomedicines10071684[91] Debard G, De Witte N, Sels R, et al. Making wearable technology available for mental healthcare through an online platform with stress detection algorithms: The carewear project. J Sens, 2020, 2020, 8846077 doi: 10.1155/2020/8846077[92] Chattopadhyay S, Das R. Comparing heart rate variability with polar H10 sensor and pulse rate variability with LYFAS: A novel study. J Biomed Eng Technol, 2021, 9, 1 doi: 10.12691/jbet-9-1-1[93] Rahman M J, Morshed B I, Harmon B, et al. A pilot study towards a smart-health framework to collect and analyze biomarkers with low-cost and flexible wearables. Smart Health, 2022, 23, 100249 doi: 10.1016/j.smhl.2021.100249[94] Mirlou F, Abbasiasl T, Mirzajani H, et al. Continuous glycemic monitoring enabled by a Wi-Fi energy-harvesting wearable sweat-sensing patch. Adv Mater Technol, 2024, 9, 2301583 doi: 10.1002/admt.202301583[95] Spigulis J, Gailite L, Lihachev A, et al. Simultaneous recording of skin blood pulsations at different vascular depths by multiwavelength photoplethysmography. Appl Opt, 2007, 46, 1754 doi: 10.1364/AO.46.001754[96] Xu H H, Liu J, Zhang J, et al. Flexible organic/inorganic hybrid near-infrared photoplethysmogram sensor for cardiovascular monitoring. Adv Mater, 2017, 29, 1700975 doi: 10.1002/adma.201700975[97] Wang S P, Yan X J, Cheng Z, et al. Highly efficient near-infrared delayed fluorescence organic light emitting diodes using a phenanthrene-based charge-transfer compound. Angew Chem Int Ed, 2015, 54, 13068 doi: 10.1002/anie.201506687[98] Hu S Q, Lin H G, Zhang Q Q, et al. Short-term HRV detection and human fatigue state analysis based on optical fiber sensing technology. Sensors, 2022, 22, 6940 doi: 10.3390/s22186940[99] Shin J H, Park K S. HRV analysis and blood pressure monitoring on weighing scale using BCG. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2012, 3789 doi: 10.1109/embc.2012.6346792[100] Zhang H Y, Wang Z, Teng C X, et al. Wearable cardiorespiratory sensor for real-time monitoring with smartphone integration. IEEE Trans Instrum Meas, 2023, 73, 7000710 doi: 10.1109/tim.2023.3338709[101] Lyu W M, Yuan W H, Yu J X, et al. Non-contact short-term heart rate variability analysis under paced respiration based on a robust fiber optic sensor system. IEEE Trans Instrum Meas, 2024, 73, 7001813 doi: 10.1109/tim.2023.3346511[102] Chen S, Qi J, Fan S, et al. Flexible wearable sensors for cardiovascular health monitoring. Adv Healthc Mater, 2021, 10, 2100116 doi: 10.1002/adhm.202100116[103] Luo W J, Sharma V, Young D J. A paper-based flexible tactile sensor array for low-cost wearable human health monitoring. J Microelectromech Syst, 2020, 29, 825 doi: 10.1109/JMEMS.2020.3011498[104] Luo W J, Sharma V, Young D J. A sock-embedded wireless sensing system employing paper-substrate-based MEMS tactile sensors array and low-power ASIC for accurate and comfortable AFib monitoring. IEEE Sens J, 2023, 23, 31050 doi: 10.1109/JSEN.2023.3329100[105] Kwak Y H, Kim W, Park K B, et al. Flexible heartbeat sensor for wearable device. Biosens Bioelectron, 2017, 94, 250 doi: 10.1016/j.bios.2017.03.016[106] Chen C M, Anastasova S, Zhang K, et al. Towards wearable and flexible sensors and circuits integration for stress monitoring. IEEE J Biomed Health Inform, 2019, 24, 2208 doi: 10.1109/jbhi.2019.2957444[107] Wang R X, Mu L W, Bao Y K, et al. Holistically engineered polymer–polymer and polymer–ion interactions in biocompatible polyvinyl alcohol blends for high-performance triboelectric devices in self-powered wearable cardiovascular monitorings. Adv Mater, 2020, 32, 2002878 doi: 10.1002/adma.202002878[108] Venkatesan C, Karthigaikumar P, Paul A, et al. ECG signal preprocessing and SVM classifier-based abnormality detection in remote healthcare applications. IEEE Access, 2018, 6, 9767 doi: 10.1109/ACCESS.2018.2794346[109] Giannakakis G, Grigoriadis D, Giannakaki K, et al. Review on psychological stress detection using biosignals. IEEE Trans Affect Comput, 2022, 13, 440 doi: 10.1109/TAFFC.2019.2927337[110] Benchekroun M, Chevallier B, Istrate D, et al. Preprocessing methods for ambulatory HRV analysis based on HRV distribution, variability and characteristics (DVC). Sensors, 2022, 22, 1984 doi: 10.3390/s22051984[111] Niu H S, Li H, Gao S, et al. Perception-to-cognition tactile sensing based on artificial-intelligence-motivated human full-skin bionic electronic skin. Advanced Materials, 2022, 34, 2202622 doi: 10.1002/adma.202202622[112] Haque Y, Zawad R S, Rony C S A, et al. State-of-the-art of stress prediction from heart rate variability using artificial intelligence. Cogn Comput, 2024, 16, 455 doi: 10.1007/s12559-023-10200-0[113] Elahi N, McNally K, Ganti L, et al. Integrating telemedicine training into the graduate medical education curriculum. Acad Med Surg, 2024, 91883 doi: 10.62186/001c.91883[114] Kim H G, Cheon E J, Bai D S, et al. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig, 2018, 15, 235 doi: 10.30773/pi.2017.08.17[115] Stroop J R. Studies of interference in serial verbal reactions. J Exp Psychol, 1935, 18, 643 doi: 10.1037/h0054651[116] Tanaka M, Mizuno K, Tajima S, et al. Central nervous system fatigue alters autonomic nerve activity. Life Sci, 2009, 84, 235 doi: 10.1016/j.lfs.2008.12.004[117] Escorihuela R M, Capdevila L, Castro J R, et al. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med, 2020, 18, 4 doi: 10.1186/s12967-019-02184-z[118] Patel M, Lal S K L, Kavanagh D, et al. Applying neural network analysis on heart rate variability data to assess driver fatigue. Expert Syst Appl, 2011, 38, 7235 doi: 10.1016/j.eswa.2010.12.028[119] Schmitt L, Regnard J, Desmarets M, et al. Fatigue shifts and scatters heart rate variability in elite endurance athletes. PLoS One, 2013, 8, e71588 doi: 10.1371/journal.pone.0071588[120] Rios-Aguilar S, Merino J L M, Millán Sánchez A, et al. Variation of the heartbeat and activity as an indicator of drowsiness at the wheel using a smartwatch. Int J Interact Multimed Artif Intell, 2015, 3, 96 doi: 10.9781/ijimai.2015.3313[121] Stephens J, Moscou-Jackson G, Allen J K. Young adults, technology, and weight loss: a focus group study. J Obes, 2015, 379769 doi: 10.1155/2015/379769[122] Pratap A, Steinhubl S, Neto E C, et al. Changes in continuous, long-term heart rate variability and individualized physiological responses to wellness and vacation interventions using a wearable sensor. Front Cardiovasc Med, 2020, 7, 120 doi: 10.3389/fcvm.2020.00120[123] Uddin S D, Hossain M S, Islam S M M, et al. Heart rate variability-based obstructive sleep apnea events classification using microwave Doppler radar. IEEE J Electromagn RF Microw Med Biol, 2021, 7, 416 doi: 10.1109/jerm.2023.3317304[124] Kim J, Kantharaju P, Yi H, et al. Soft wearable flexible bioelectronics integrated with an ankle-foot exoskeleton for estimation of metabolic costs and physical effort. NPJ Flex Electron, 2023, 7, 3 doi: 10.1038/s41528-023-00239-2[125] Pei Z J, Shi M H, Guo J P, et al. Heart rate variability based prediction of personalized drug therapeutic response: The present status and the perspectives. Curr Top Med Chem, 2020, 20, 1640 doi: 10.2174/1568026620666200603105002[126] Jovic A, Bogunovic N. Electrocardiogram analysis using a combination of statistical, geometric, and nonlinear heart rate variability features. Artif Intell Med, 2011, 51, 175 doi: 10.1016/j.artmed.2010.09.005[127] Cho H M, Park H, Dong S Y, et al. Ambulatory and laboratory stress detection based on raw electrocardiogram signals using a convolutional neural network. Sensors, 2019, 19, 4408 doi: 10.3390/s19204408[128] Aishwarya S. Introduction to neural network: Convolutional neural network. Analytics Vidhya, 2020[129] Kang M, Shin S, Jung J, et al. Classification of mental stress using CNN-LSTM algorithms with electrocardiogram signals. J Healthc Eng, 2021, 2021, 9951905 doi: 10.1155/2021/9951905 -
Proportional views





 Feifei Yin received her Master's degree in the School of Information Science and Engineering from the University of Jinan, China in 2021. She finished her Ph.D. dgree in the Department of Electronics Engineering at Kwangwoon University in 2024, South Korea. Now, she is a researcher working in the East China Institute of Photo-Elextro ICs, China. Her current research focuses on gas sensing devices and flexible electronic devices.
Feifei Yin received her Master's degree in the School of Information Science and Engineering from the University of Jinan, China in 2021. She finished her Ph.D. dgree in the Department of Electronics Engineering at Kwangwoon University in 2024, South Korea. Now, she is a researcher working in the East China Institute of Photo-Elextro ICs, China. Her current research focuses on gas sensing devices and flexible electronic devices. Yang Li has been a professor at the School of Microelectronics at the Shandong University, China since 2023. He received his Ph.D. degree in the Department of Electronics Engineering at Kwangwoon University, South Korea in 2015. He conducted his Postdoc research in the department of Electronic Engineering at Kwangwoon University, Korea from 2015 to 2016. He has published over 100 peer-reviewed journal papers and has been authorized for over 40 Chinese and Korean patents. His research interests include advanced semiconductor fabrication, nanostructured flexible materials, gas sensors, and memristors.
Yang Li has been a professor at the School of Microelectronics at the Shandong University, China since 2023. He received his Ph.D. degree in the Department of Electronics Engineering at Kwangwoon University, South Korea in 2015. He conducted his Postdoc research in the department of Electronic Engineering at Kwangwoon University, Korea from 2015 to 2016. He has published over 100 peer-reviewed journal papers and has been authorized for over 40 Chinese and Korean patents. His research interests include advanced semiconductor fabrication, nanostructured flexible materials, gas sensors, and memristors.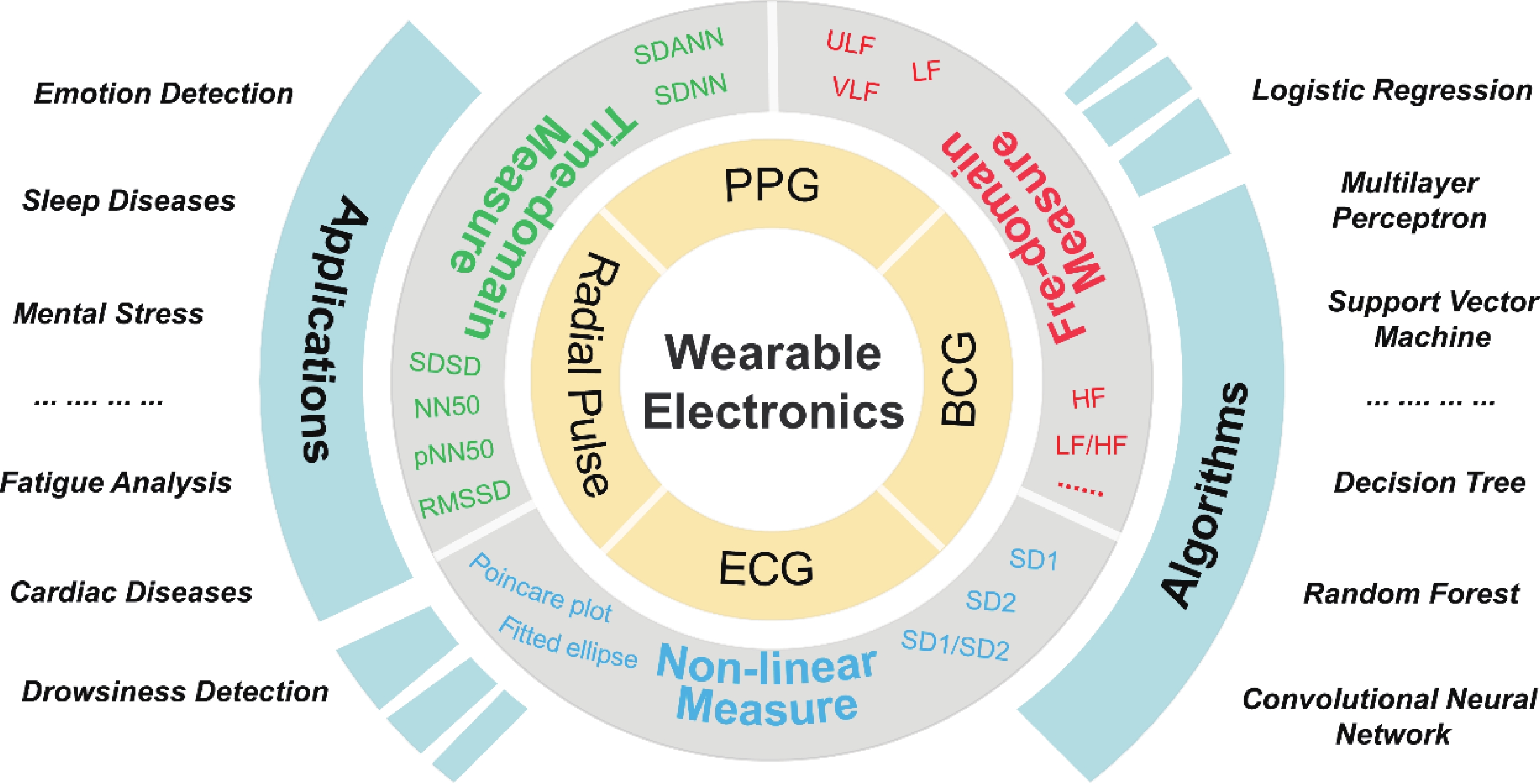
 DownLoad:
DownLoad: