| Citation: |
Shanshan Yu, Yichao Gan, Feifan Song, Qiongzhang Wang, Hao Tang, Zhao Li. A miniaturized wireless electrical impedance myography platform for the long-term adaptive muscle fatigue monitoring[J]. Journal of Semiconductors, 2025, 46(10): 102601. doi: 10.1088/1674-4926/25020029
****
S S Yu, Y C Gan, F F Song, Q Z Wang, H Tang, and Z Li, A miniaturized wireless electrical impedance myography platform for the long-term adaptive muscle fatigue monitoring[J]. J. Semicond., 2025, 46(10), 102601 doi: 10.1088/1674-4926/25020029
|
A miniaturized wireless electrical impedance myography platform for the long-term adaptive muscle fatigue monitoring
DOI: 10.1088/1674-4926/25020029
CSTR: 32376.14.1674-4926.25020029
More Information-
Abstract
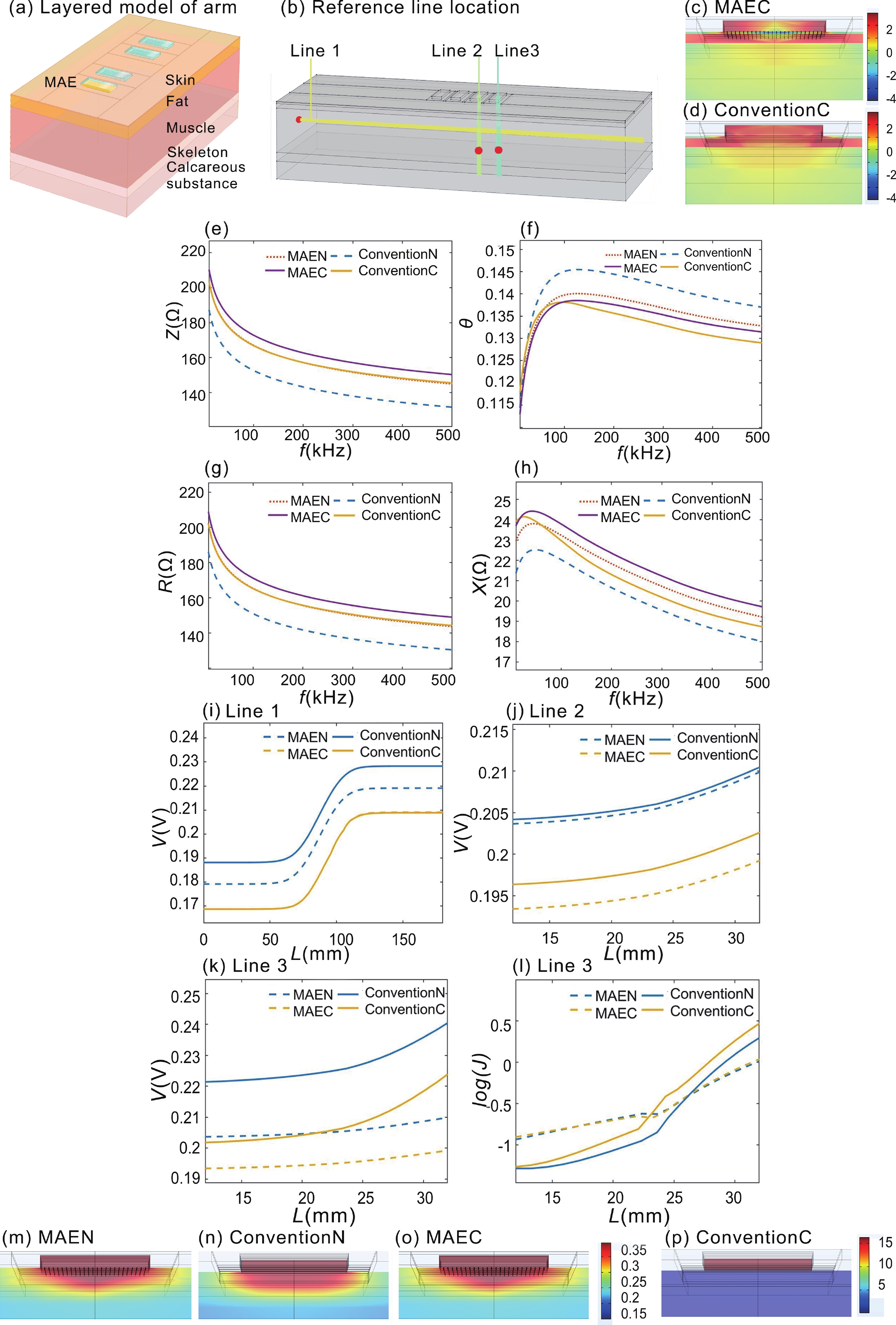
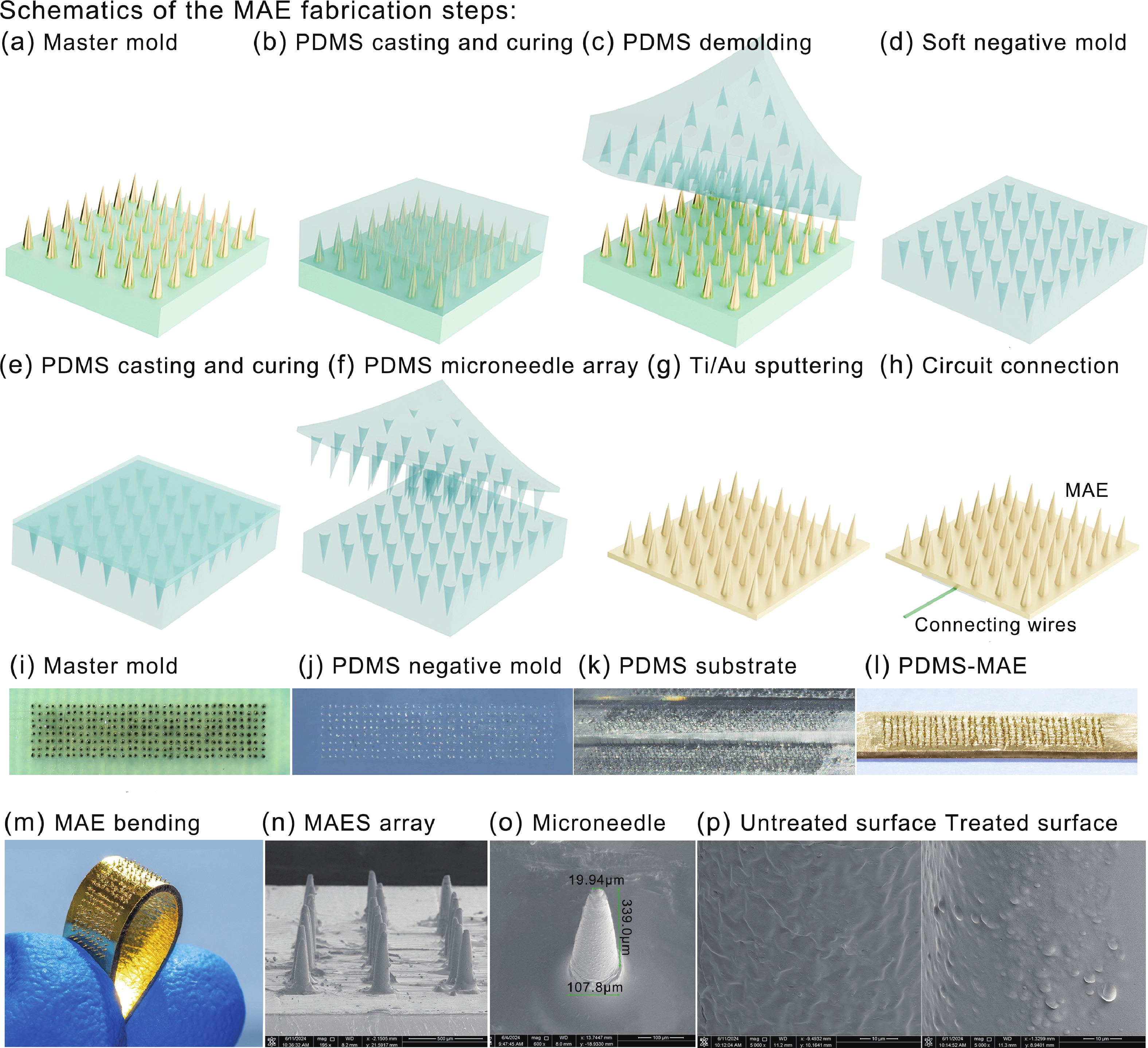
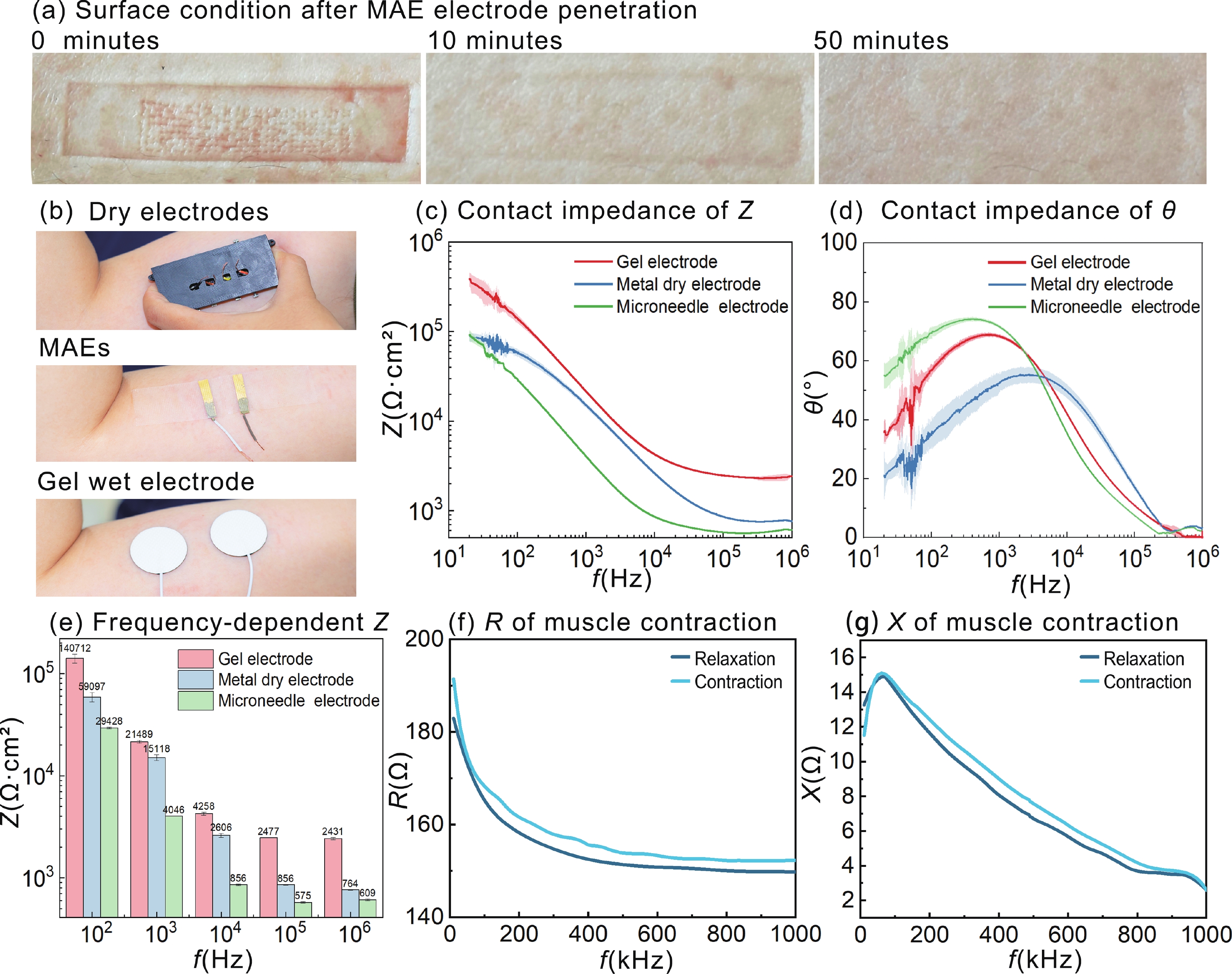
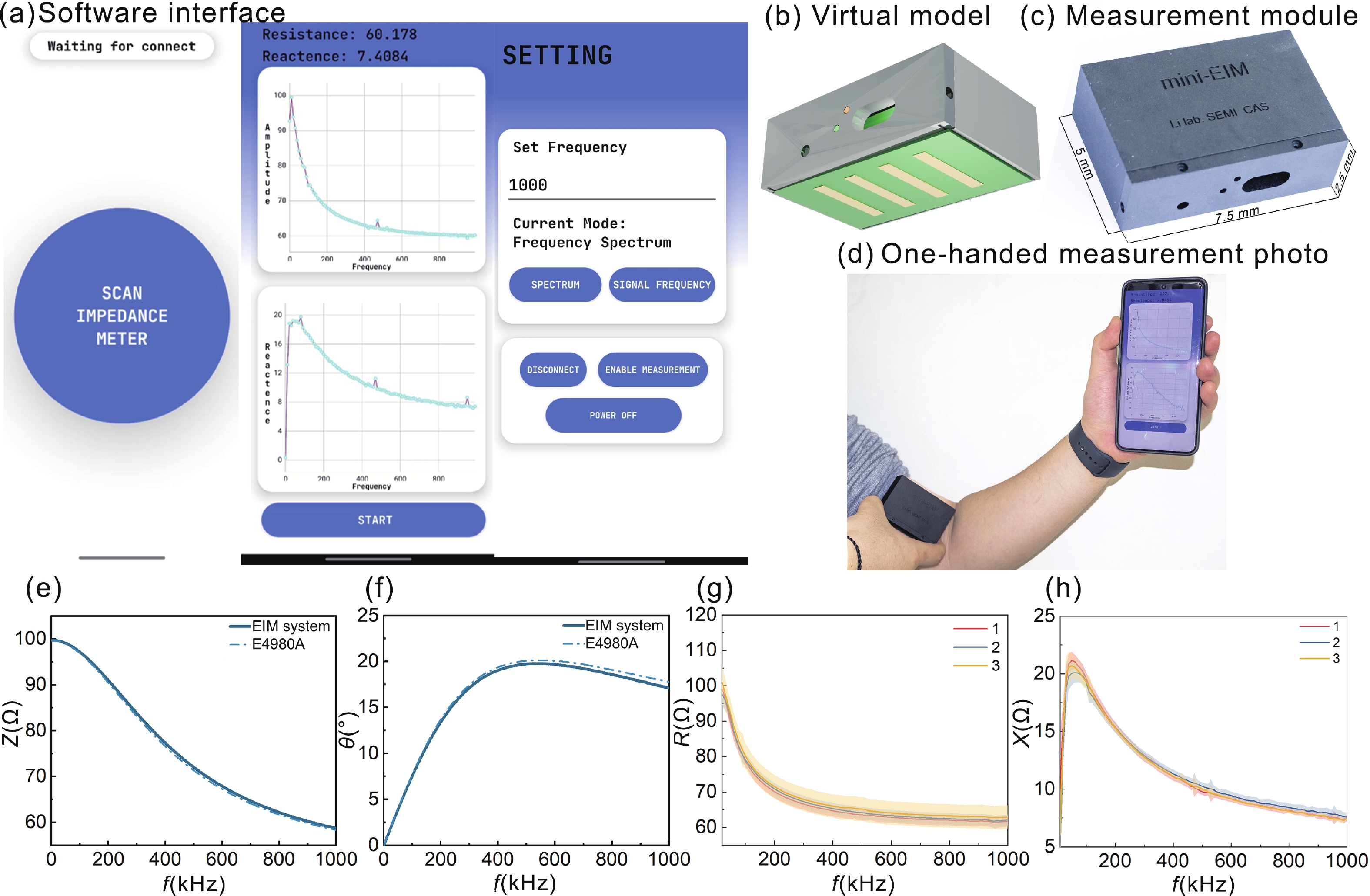
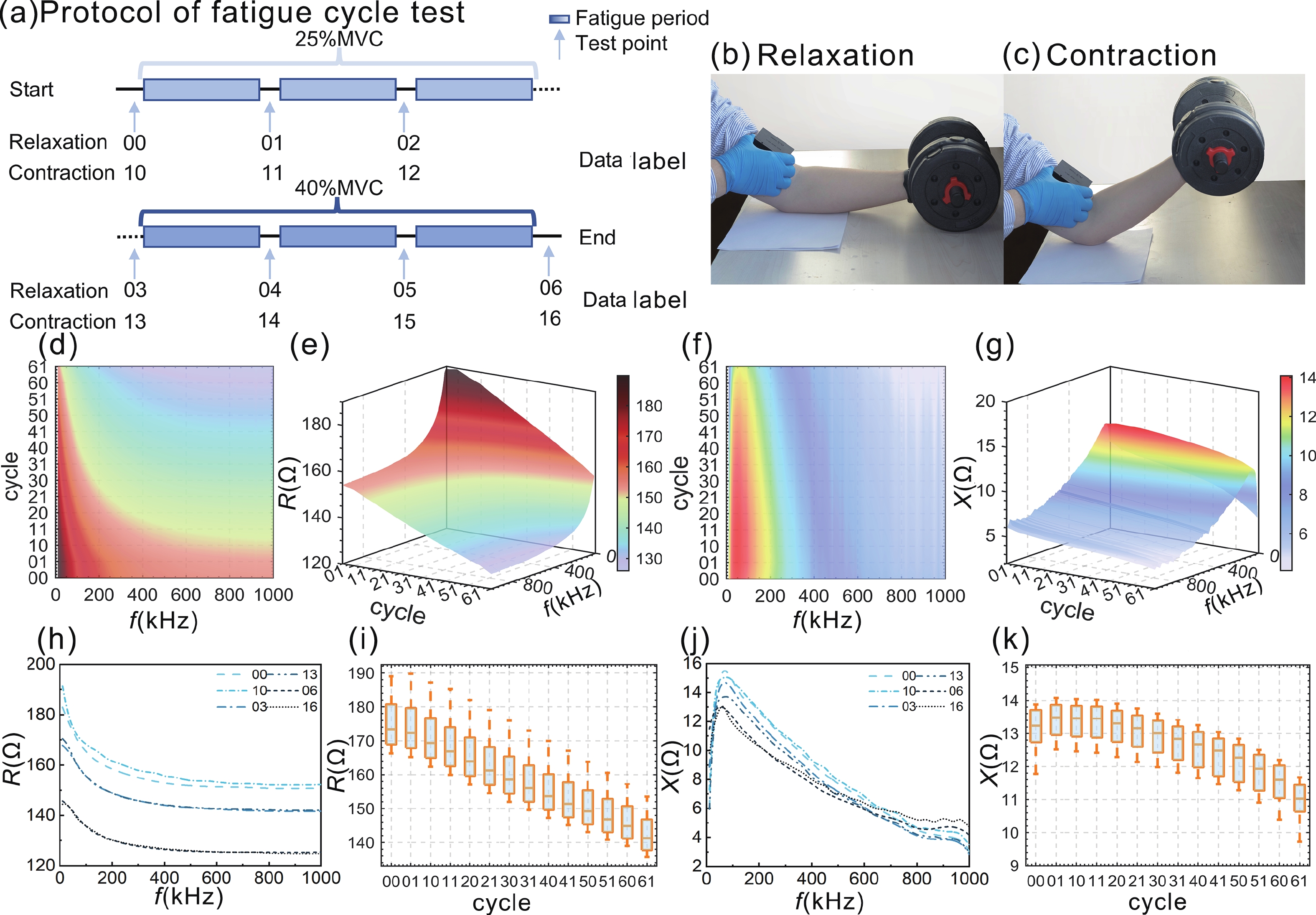
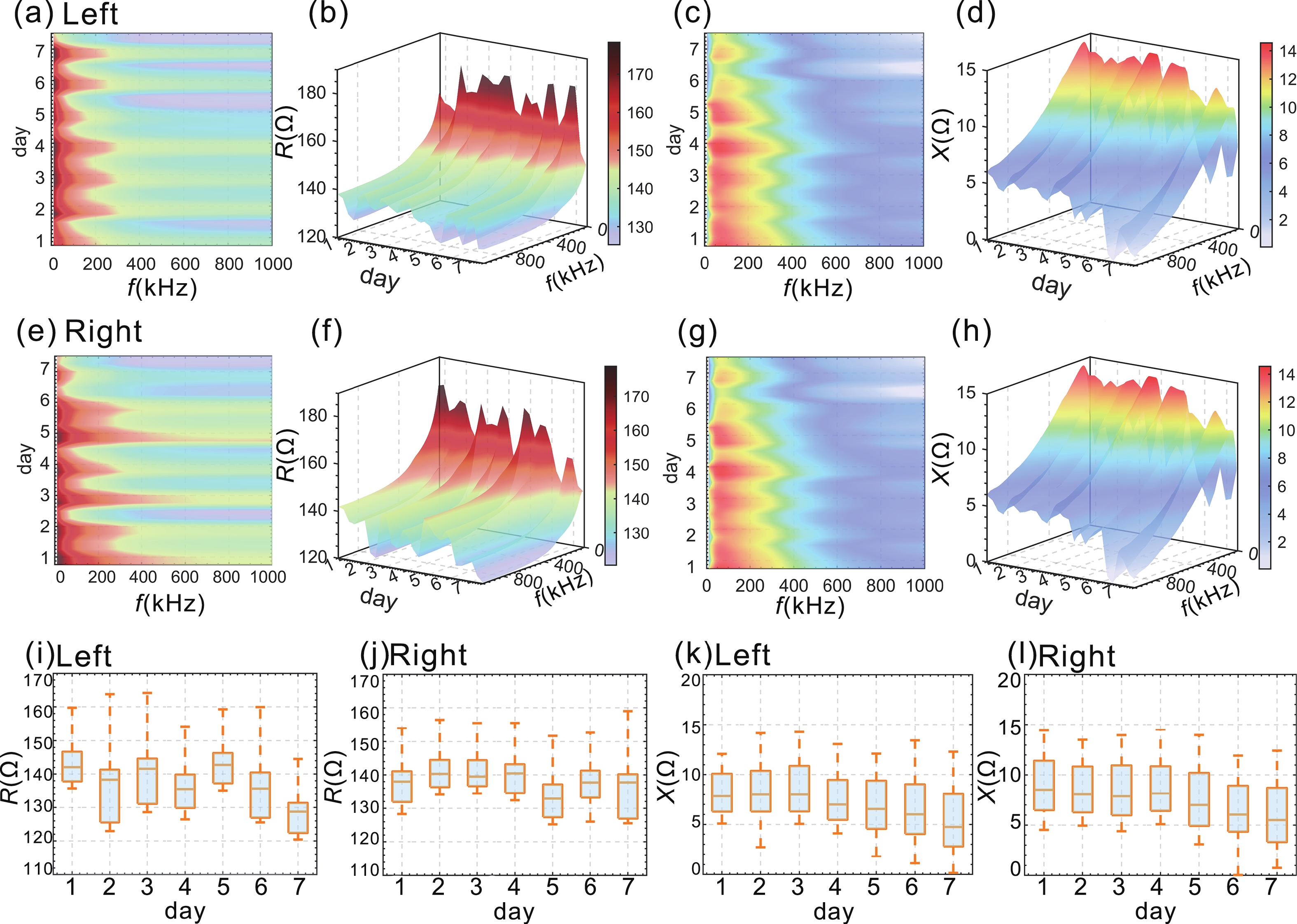
Accurate quantification of exercise interventions and changes in muscle function is essential for personalized health management. Electrical impedance myography (EIM) technology offers an innovative, noninvasive, painless, and easy-to-perform solution for muscle health monitoring. However, current EIM platforms face a number of limitations, including large device size, wired connections, and instability of the electrode-skin interface, which limit their applicability for monitoring muscle movement. In this study, a miniature wireless EIM platform with a user-friendly smartphone app is proposed and developed. The miniature, wireless, multi-frequency (20 kHz−1 MHz) EIM platform is equipped with flexible microneedle array electrodes (MAE). The advantages of MAEs over conventional electrodes were demonstrated by physical field modeling simulations and skin-electrode contact impedance comparison tests. The smartphone APP was developed to wirelessly operate the EIM platform, and to transmit and process real-time muscle impedance data. To validate its effectiveness, a seven-day adaptive fatigue training study was conducted, which demonstrated that the EIM platform was able to detect muscle adaptations and serve as a reliable indicator of fatigue. This study presents an innovative approach to applying EIM technology to muscle health monitoring and exercise testing, thereby advancing the development of personalized health management and athletic performance assessment. -
References
[1] Enoka R M, Duchateau J. Muscle fatigue: What, why and how it influences muscle function. J Physiol, 2008, 586(1), 11 doi: 10.1113/jphysiol.2007.139477[2] Li N, Zhou R, Krishna B, et al. Non-invasive techniques for muscle fatigue monitoring: A comprehensive survey. ACM Comput Surv, 2024, 56(9), 1 doi: 10.1145/3648679[3] Qin X K, Zhong B W, Lv S X, et al. A zero-voltage-writing artificial nervous system based on biosensor integrated on ferroelectric tunnel junction. Adv Mater, 2024, 36(32), e2404026 doi: 10.1002/adma.202404026[4] Guven A E, Chiapparelli E, Camino−Willhuber G, et al. Assessing paraspinal muscle atrophy with electrical impedance myography: Limitations and insights. J Orthop Res, 2024, 42(9), 2072 doi: 10.1002/jor.25848[5] Schrunder A F, Rodriguez S, Rusu A. A finite element analysis and circuit modelling methodology for studying electrical impedance myography of human limbs. IEEE T-BME, 2022, 69(1), 244 doi: 10.1109/TBME.2021.3091884[6] Kapravchuk V, Briko A, Kobelev A, et al. An approach to using electrical impedance myography signal sensors to assess morphofunctional changes in tissue during muscle contraction. Biosensors, 2024, 14(2), 76 doi: 10.3390/bios14020076[7] Chrzanowski S M, Nagy J A, Pandeya S, et al. Electrical impedance myography correlates with functional measures of disease progression in D2-mdx mice and boys with Duchenne muscular dystrophy. J Neuromuscul Dis, 2023, 10(1), 81 doi: 10.3233/JND-210787[8] Rutkove S B. Electrical impedance myography: Background, current state, and future directions. Muscle Nerve, 2009, 40(6), 936 doi: 10.1002/mus.21362[9] Critcher S, Freeborn T J. Short-term evaluation of dry electrodes fabricated using flexible printed circuits processes for bioimpedance measurements. 2020 SoutheastCon, 2020, 1 doi: 10.1109/SoutheastCon44009.2020.9249690[10] Li Z, Li Y, Liu M S, et al. Microneedle electrode array for electrical impedance myography to characterize neurogenic myopathy. Ann Biomed Eng, 2016, 44(5), 1566 doi: 10.1007/s10439-015-1466-5[11] Li Z, Chen L F, Zhu Y, et al. Handheld electrical impedance myography probe for assessing carpal tunnel syndrome. Ann Biomed Eng, 2017, 45(6), 1572 doi: 10.1007/s10439-017-1819-3[12] Huang L K, Huang L N, Gao Y M, et al. Electrical impedance myography applied to monitoring of muscle fatigue during dynamic contractions. IEEE Access, 2020, 8, 13056 doi: 10.1109/ACCESS.2020.2965982[13] Kusche R, Oltmann A, Rostalski P. A wearable dual-channel bioimpedance spectrometer for real-time muscle contraction detection. IEEE Sens J, 2024, 24(7), 11316 doi: 10.1109/JSEN.2024.3359284[14] Qin X K, Zhong B W, Xu H, et al. Manufacturing high−performance flexible sensors via advanced patterning techniques. Int J Extrem Manuf, 2025, 7, 032003 doi: 10.1088/2631-7990/ada857[15] Ren L, Liu B, Zhou W, et al. A mini review of microneedle array electrode for bio-signal recording: A review. IEEE Sens J, 2020, 20(2), 577 doi: 10.1109/JSEN.2019.2944847[16] Wang Z Y, Huang D, Li J S, et al. Ag/AgCl-covered flexible microneedle array dry electrode for electrocardiography recording. 2023 IEEE 18th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), 2023, 74 doi: 10.1109/NEMS57332.2023.10190967[17] Wolf M P, Salieb-Beugelaar G B, Hunziker P. PDMS with designer functionalities: Properties, modifications strategies, and applications. Prog Polym Sci, 2018, 83, 97 doi: 10.1016/j.progpolymsci.2018.06.001[18] Wang R X, Bai J X, Zhu X H, et al. A PDMS-based microneedle array electrode for long-term ECG recording. Biomed Microdevices, 2022, 24(3), 27 doi: 10.1007/s10544-022-00626-y[19] Reddy J N. An introduction to the finite element method. J Press Vessel Technol, 1989, 111(3), 348 doi: 10.1115/1.3265687[20] Tang X, Dong Y Z, Li Q G, et al. Using microneedle array electrodes for non-invasive electrophysiological signal acquisition and sensory feedback evoking. Front Bioeng Biotechnol, 2023, 11, 1238210 doi: 10.3389/fbioe.2023.1238210[21] Li J S, Ma Y D, Huang D, et al. High-performance flexible microneedle array as a low-impedance surface biopotential dry electrode for wearable electrophysiological recording and polysomnography. Nanomicro Lett, 2022, 14(1), 132 doi: 10.1007/s40820-022-00870-0[22] Singh O P, El-Badawy I M, Sundaram S, et al. Microneedle electrodes: Materials, fabrication methods, and electrophysiological signal monitoring-narrative review. Biomed Microdevices, 2025, 27(1), 9 doi: 10.1007/s10544-024-00732-z[23] Liu Z J, Xu X Y, Huang S, et al. Multichannel microneedle dry electrode patches for minimally invasive transdermal recording of electrophysiological signals. Microsyst Nanoeng, 2024, 10, 72 doi: 10.1038/s41378-024-00702-8[24] Xu P, Yang X D, Ma W, et al. A study of handgrip force prediction scheme based on electrical impedance myography. IEEE J−ERM, 2023, 7(1), 90 doi: 10.1109/JERM.2023.3241769[25] Cebrián-Ponce Á, Irurtia A, Carrasco-Marginet M, et al. Electrical impedance myography in health and physical exercise: A systematic review and future perspectives. Front Physiol, 2021, 12, 740877 doi: 10.3389/fphys.2021.740877[26] Hülkenberg A, Strotmann N, Mokni A, et al. The influence of muscle contraction intensity on electrical impedance myography. 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), 2024, 1 doi: 10.1109/EMBC53108.2024.10782074[27] Byström S. Estimation of aerobic and anaerobic metabolism in isometric forearm exercise. Ups J Med Sci, 1994, 99(1), 51 doi: 10.3109/03009739409179350[28] IEEE Standard on Piezoelectricity. ANSI/IEEE Std 176−1987. New York: IEEE, 1988, 1[29] Ji H W, Wang M Y, Wang Y T, et al. Skin-integrated, biocompatible, and stretchable silicon microneedle electrode for long-term EMG monitoring in motion scenario. NPJ Flex Electron, 2023, 7, 46 doi: 10.1038/s41528-023-00279-8[30] Gao K P, Yang H J, Wang X L, et al. Soft pin-shaped dry electrode with bristles for EEG signal measurements. Sensor Actuat A−Phys, 2018, 283, 348 doi: 10.1016/j.sna.2018.09.045[31] Shiffman C A, Aaron R, Rutkove S B. Electrical impedance of muscle during isometric contraction. Physiol Meas, 2003, 24(1), 213 doi: 10.1088/0967-3334/24/1/316 -
Supplements
 25020029Supplementary_Material.pdf
25020029Supplementary_Material.pdf

-
Proportional views





 Shanshan Yu got her B.S. from University of Science and Technology Beijing in 2022. Now she is a M.D student at University of Chinese Academy of Sciences under the supervision of Prof. Zhao Li. Her research focuses on development of semiconductor biochemical sensing and detection equipment.
Shanshan Yu got her B.S. from University of Science and Technology Beijing in 2022. Now she is a M.D student at University of Chinese Academy of Sciences under the supervision of Prof. Zhao Li. Her research focuses on development of semiconductor biochemical sensing and detection equipment. Yichao Gan got his B.S. from Minzu University of China in 2025. Now he is a Ph.D. student at Institute of Semiconductors, Chinese Academy of Sciences under the supervision of Prof. Zhao Li. His research focuses on development of semiconductor biochemical sensing, detection equipment, robotics and embodied AI.
Yichao Gan got his B.S. from Minzu University of China in 2025. Now he is a Ph.D. student at Institute of Semiconductors, Chinese Academy of Sciences under the supervision of Prof. Zhao Li. His research focuses on development of semiconductor biochemical sensing, detection equipment, robotics and embodied AI. Hao Tang received the B.Eng. degree from Beijing University of Posts and Telecommunications and M.Eng. degree from Tsinghua University. He did his Ph.D. studies at Princeton University from 2016 to 2023. His research interests include developing new architectures for point-of-care diagnostics and chip-scale platforms for cellular assays. In particular, he has been interested in high throughput, pneumatic-free, and cytometry with high specificity and sensitivity. He joined the Institute of Semiconductors, Chinese Academy of Sciences as a research engineer since 2024.
Hao Tang received the B.Eng. degree from Beijing University of Posts and Telecommunications and M.Eng. degree from Tsinghua University. He did his Ph.D. studies at Princeton University from 2016 to 2023. His research interests include developing new architectures for point-of-care diagnostics and chip-scale platforms for cellular assays. In particular, he has been interested in high throughput, pneumatic-free, and cytometry with high specificity and sensitivity. He joined the Institute of Semiconductors, Chinese Academy of Sciences as a research engineer since 2024. Zhao Li received his B.Eng. degree in Communication Engineering from Wuhan University of Technology in 2012, and the Ph.D. Degree in Physical Electronics from the University of Chinese Academy of Sciences in 2017. From 2017 to 2021, he worked as a postdoctoral researcher in Ping Wang's lab at the Perelman School of Medicine, University of Pennsylvania. Since 2021, he has been with the Institute of Semiconductors, Chinese Academy of Sciences, as a full professor working on the novel biochemical sensors and medical testing equipment development.
Zhao Li received his B.Eng. degree in Communication Engineering from Wuhan University of Technology in 2012, and the Ph.D. Degree in Physical Electronics from the University of Chinese Academy of Sciences in 2017. From 2017 to 2021, he worked as a postdoctoral researcher in Ping Wang's lab at the Perelman School of Medicine, University of Pennsylvania. Since 2021, he has been with the Institute of Semiconductors, Chinese Academy of Sciences, as a full professor working on the novel biochemical sensors and medical testing equipment development.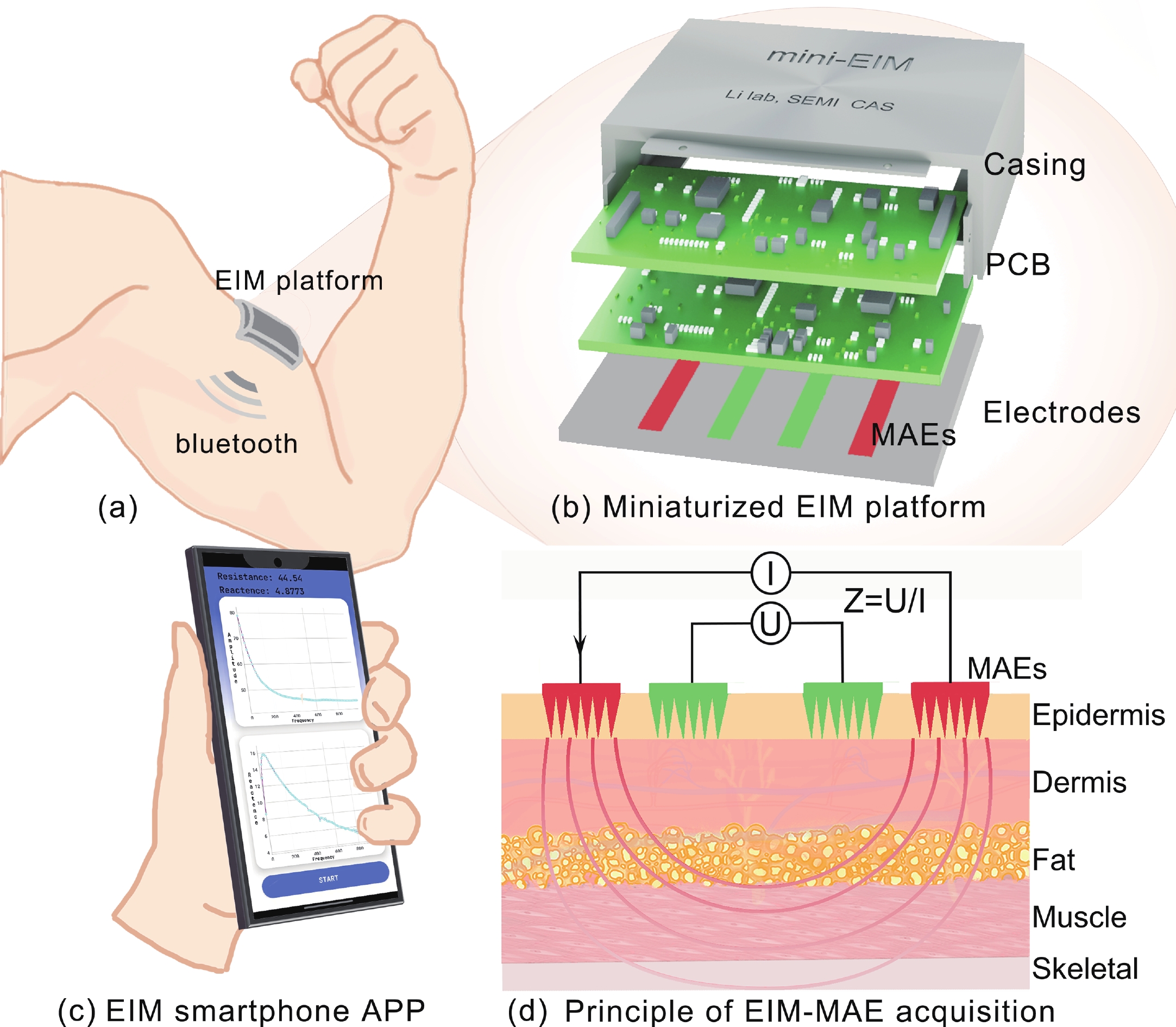
 DownLoad:
DownLoad: