| Citation: |
Yinzhong Liu, Xuechun Yang, Yun Guo, Lingchao Wang, Xiaofan Li, Hui Guo, Yiyu Qiao, Xiaotao Zhu, Lingli Cheng, Zheng Jiao. Harnessing Eu/Ce-codoped ZnO nanomaterial derived from MOF precursor for high-performance n-butanol sensing under UV activation at ambient temperature[J]. Journal of Semiconductors, 2026, In Press. doi: 10.1088/1674-4926/25070023
****
Y Z Liu, X C Yang, Y Guo, L C Wang, X F Li, H Guo, Y Y Qiao, X T Zhu, L L Cheng, and Z Jiao, Harnessing Eu/Ce-codoped ZnO nanomaterial derived from MOF precursor for high-performance n-butanol sensing under UV activation at ambient temperature[J]. J. Semicond., 2026, 47(2): 022503 doi: 10.1088/1674-4926/25070023
|
Harnessing Eu/Ce-codoped ZnO nanomaterial derived from MOF precursor for high-performance n-butanol sensing under UV activation at ambient temperature
DOI: 10.1088/1674-4926/25070023
CSTR: 32376.14.1674-4926.25070023
More Information-
Abstract
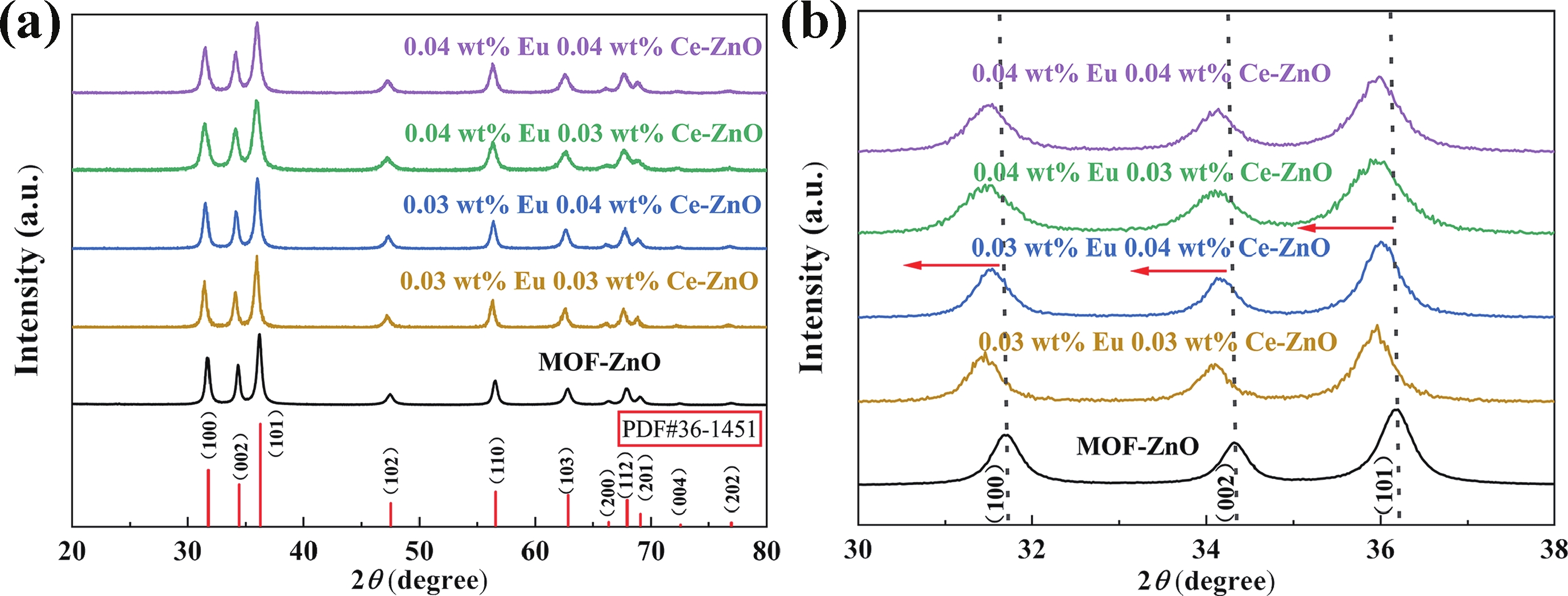
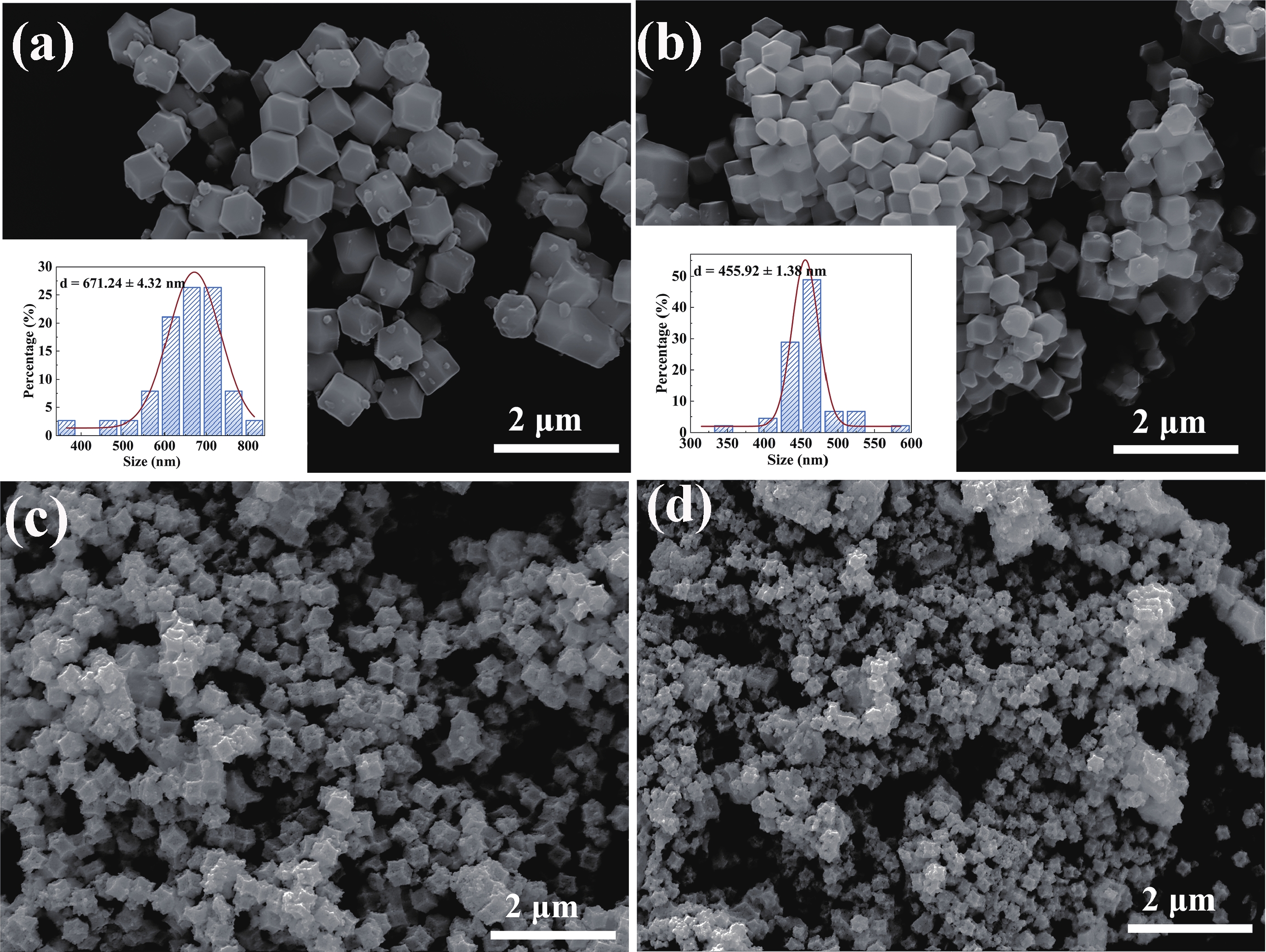
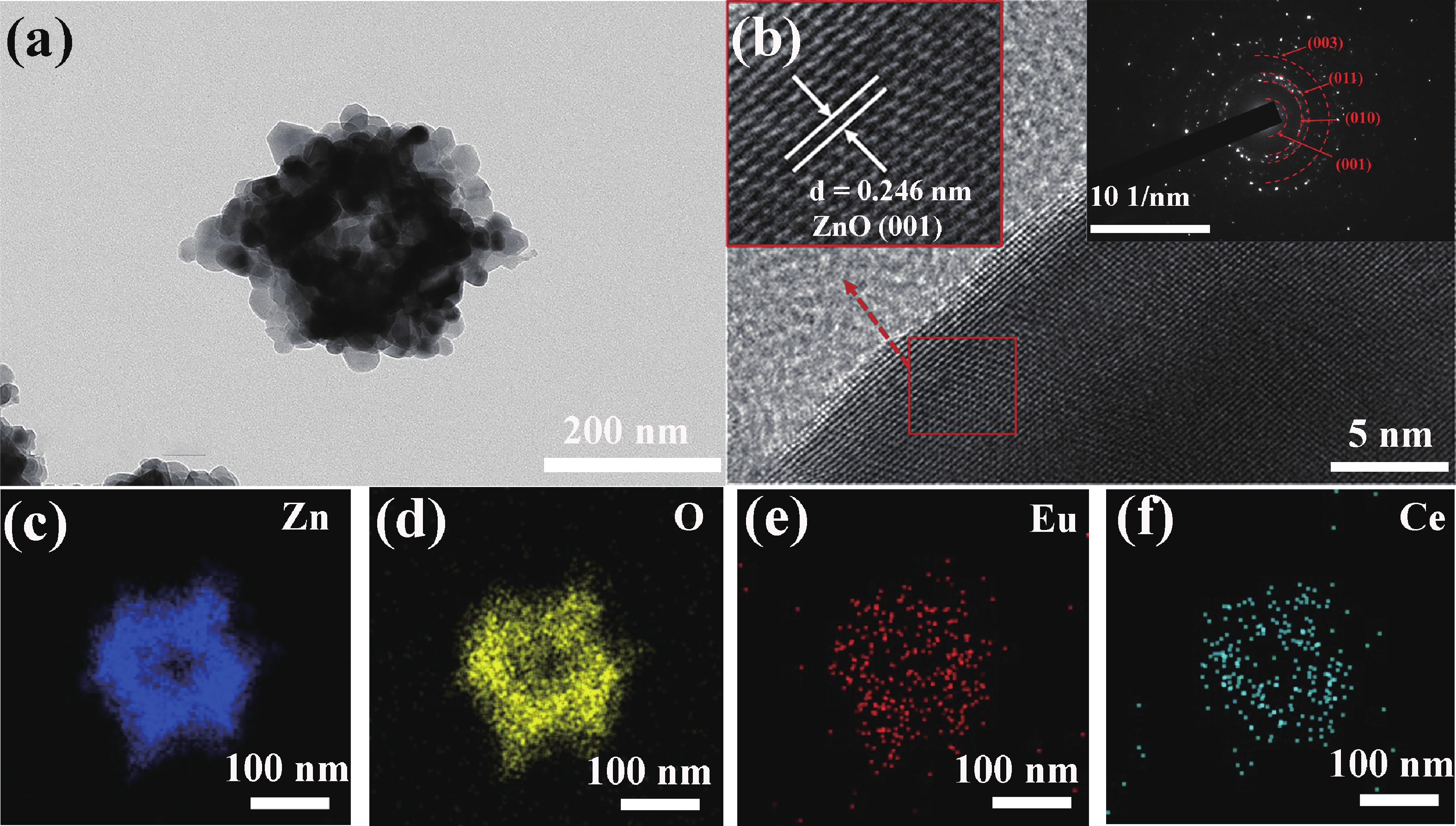
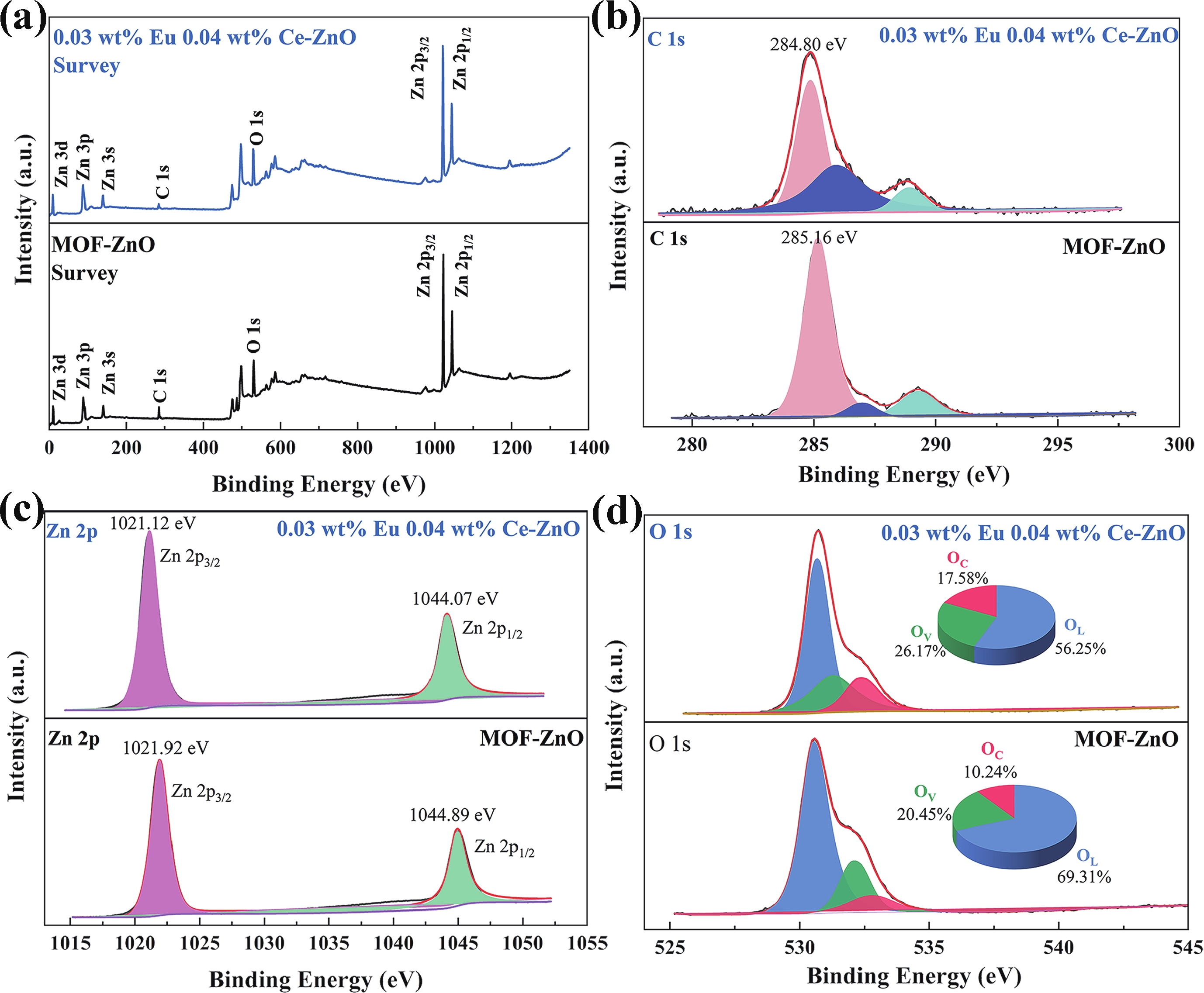
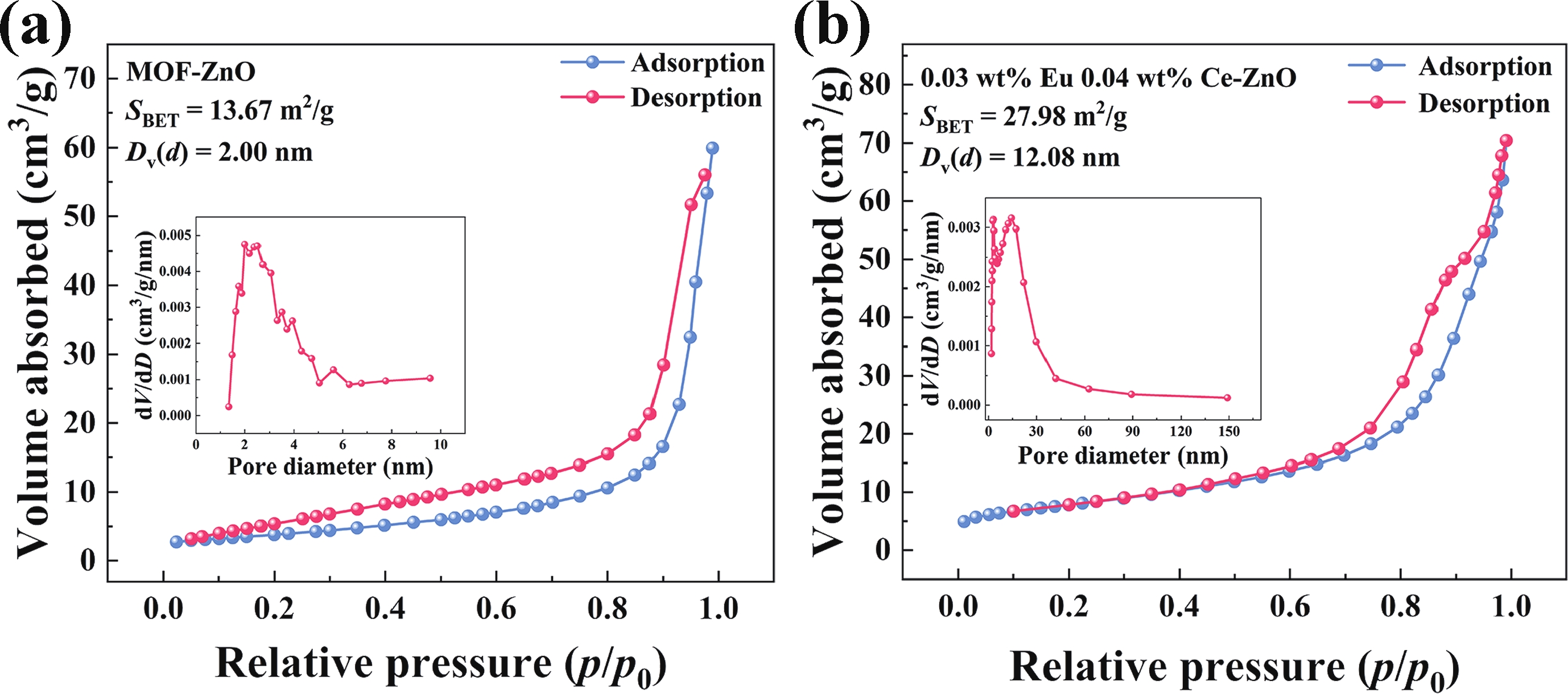
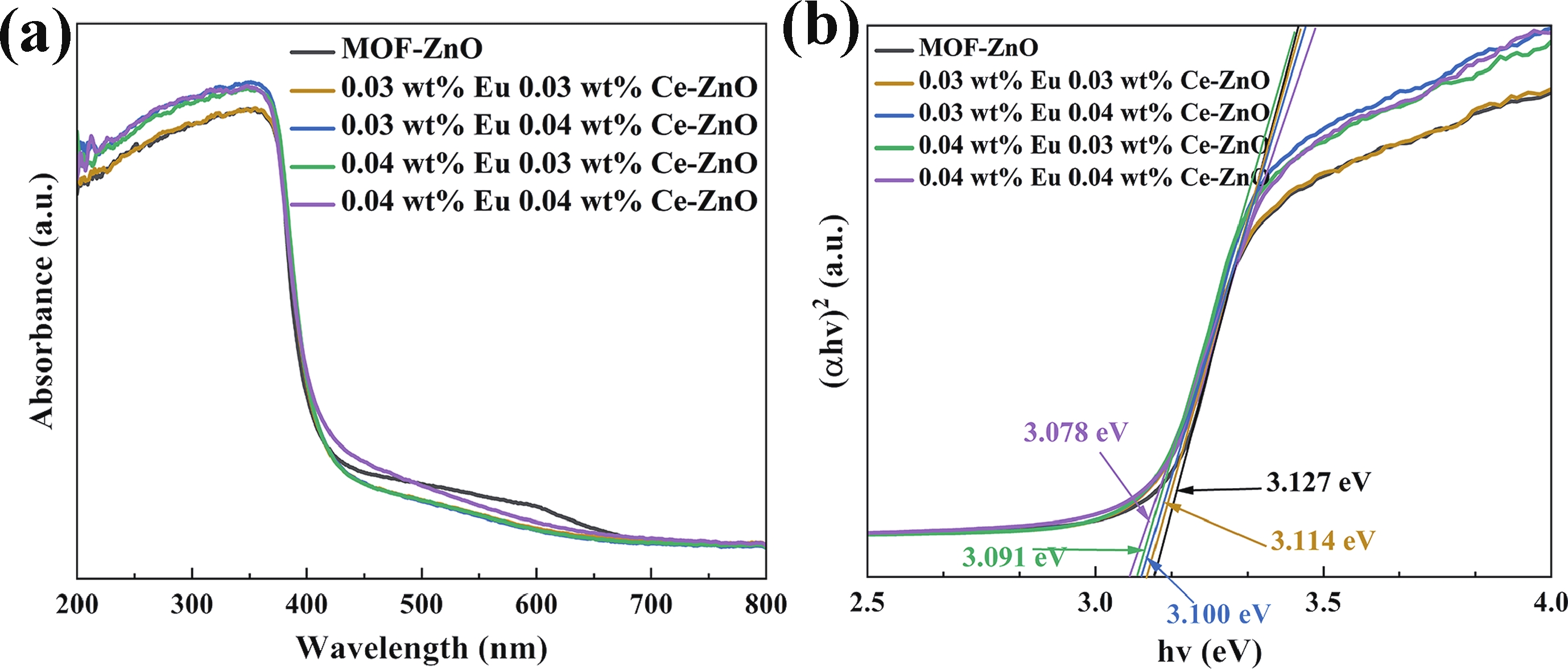
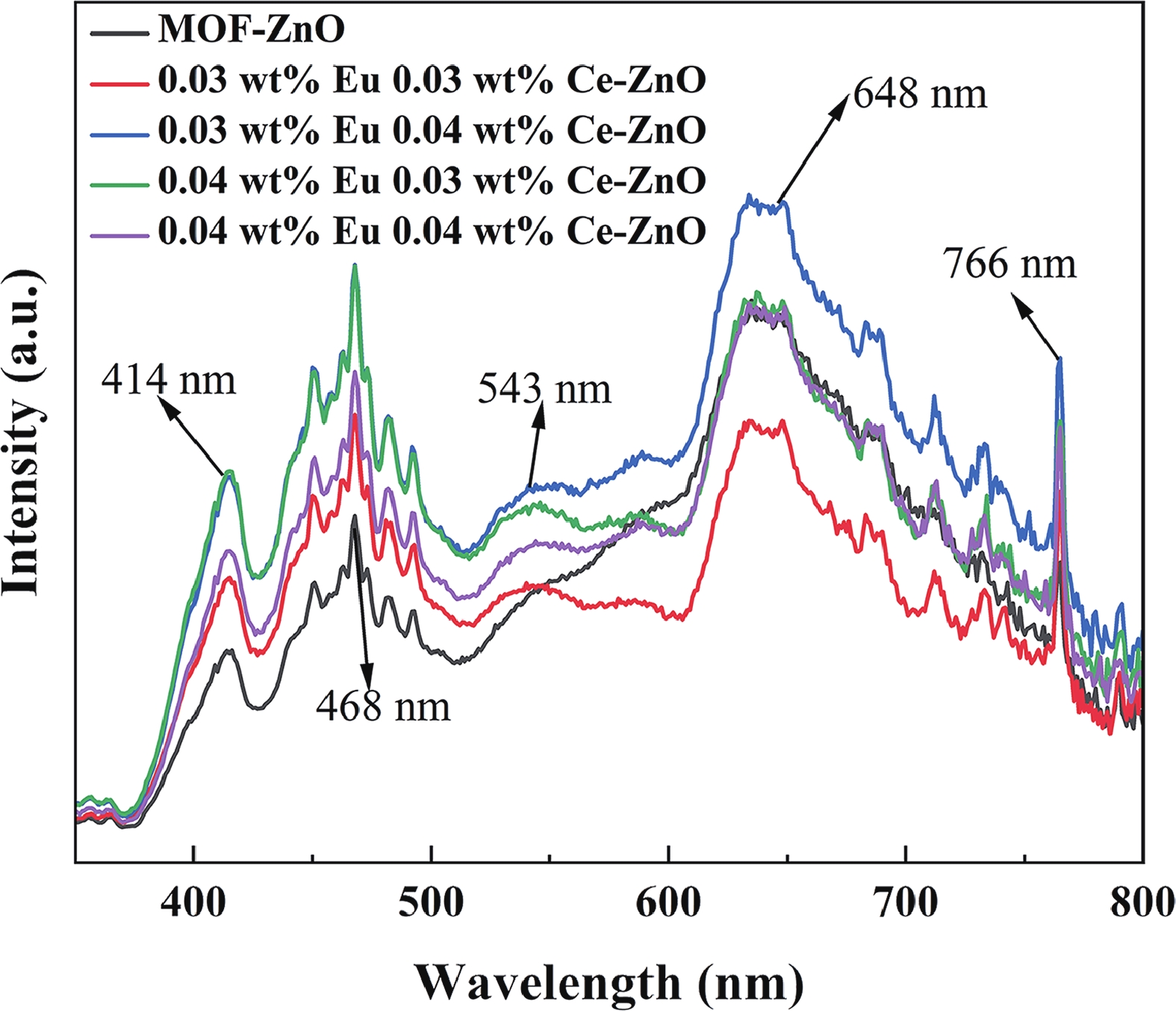
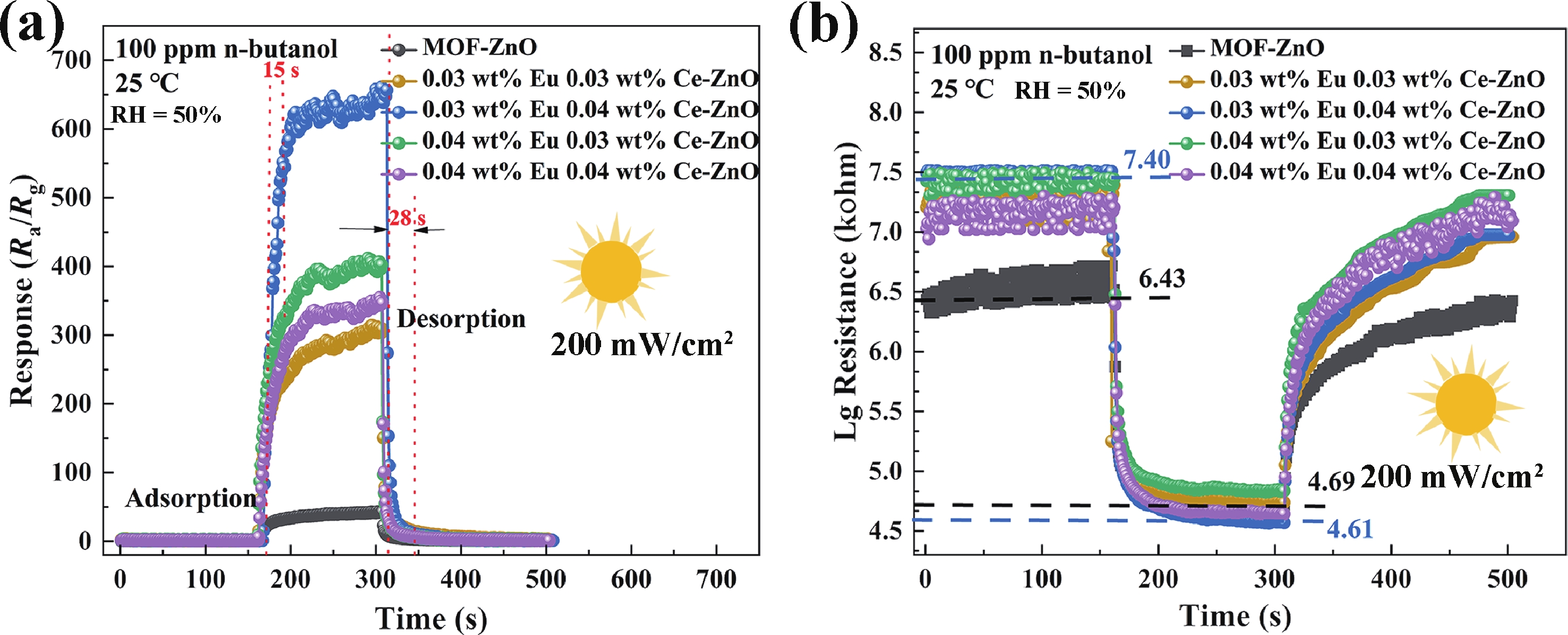
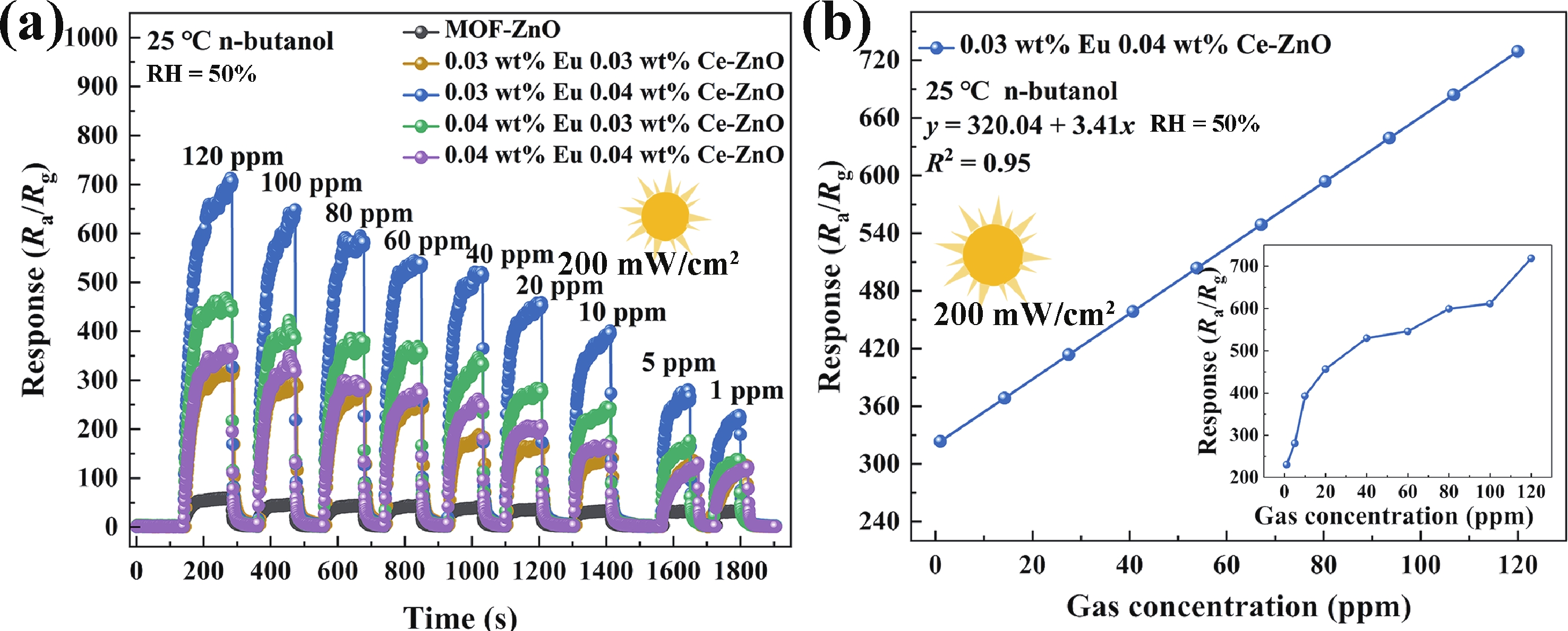
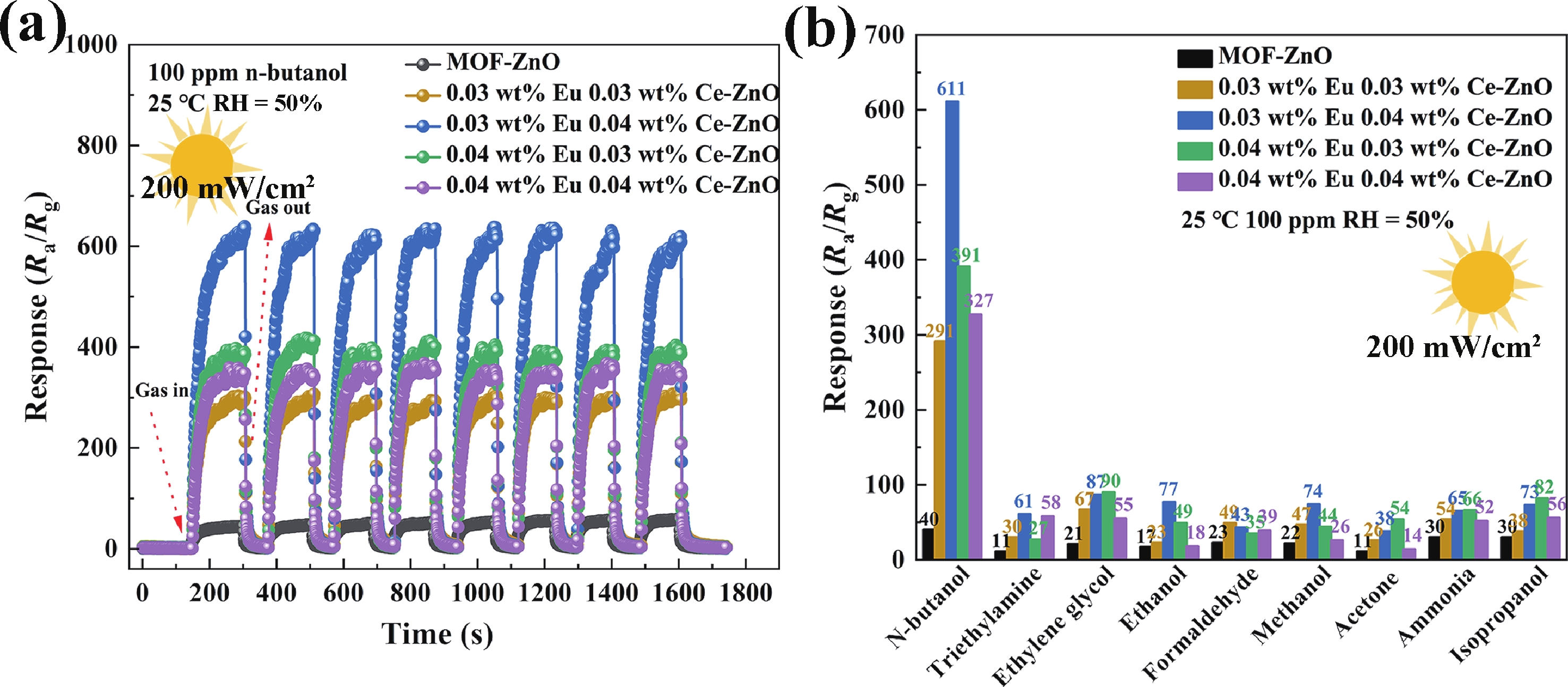
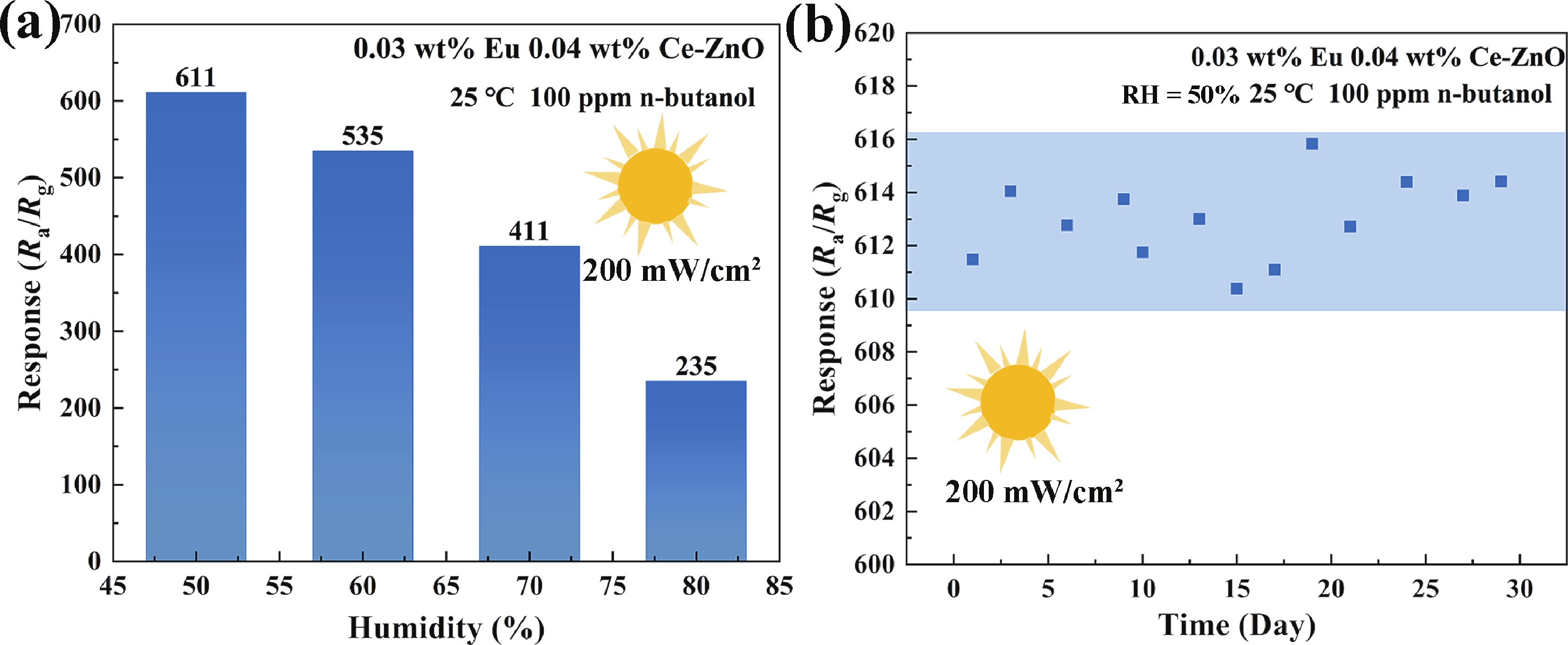
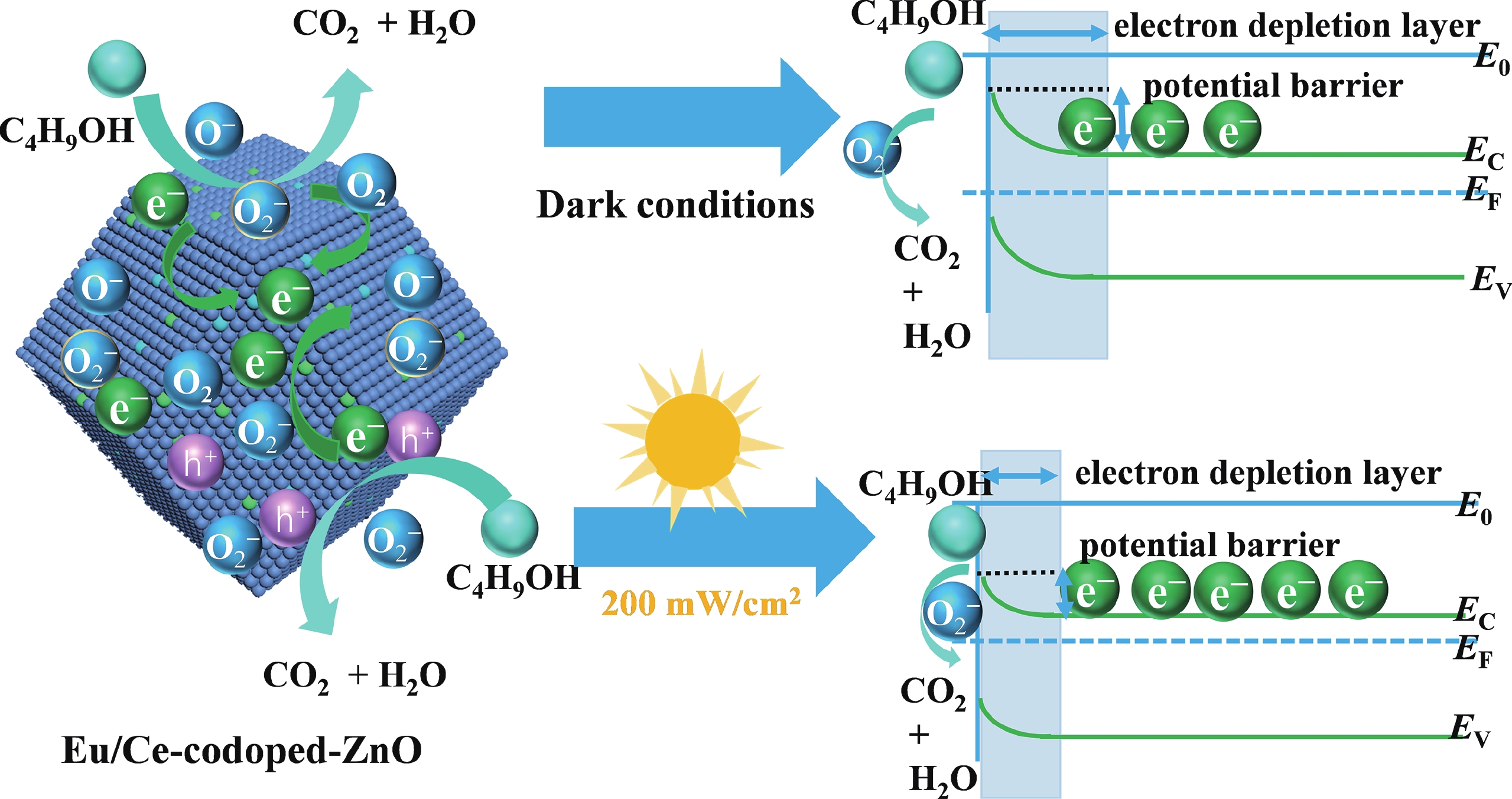
Prolonged exposure to n-butanol, a common hazardous volatile organic compound (VOC) in the environment, can lead to a broad range of adverse health effects. Therefore, detecting n-butanol safely and efficiently at low concentrations becomes critical for both environmental monitoring and human health. In this study, a novel Eu/Ce-codoped MOF-ZnO gas sensor was developed for the sensitive detection of n-butanol gas under ultraviolet activation at ambient temperature. A series of Eu/Ce-ZnO nanomaterials were synthesized via a simple co-precipitation route, by carefully designing the varied mass ratios of Eu and Ce incorporated into pristine ZnO derived from MOF precursors. The gas testing results revealed that introducing an appropriate amount of Eu and Ce would enlarge the specific surface area and enrich the oxygen vacancy content compared to pristine MOF-ZnO. Upon UV irradiation, the 0.03 wt% Eu 0.04 wt% Ce-ZnO sensor achieved a superior response of 611 for 100 ppm n-butanol at room temperature, 15.28 times higher than that of pristine MOF-ZnO (40). Furthermore, the sensor presented rapid response/recovery times (15 s/28 s) and excellent selectivity. The above contributions pave the way for the promising development of highly sensitive, ultraviolet-enhanced gas sensors for ambient temperature detection of VOCs.-
Keywords:
- Eu/Ce,
- ZnO,
- N-butanol,
- ultraviolet,
- ambient temperature
-
References
[1] Thayil R, Parne S R. Biofunctionalized magnetic nanoparticles incorporated MoS2 nanocomposite for enhanced n-butanol sensing at room temperature. Sci Rep, 2024, 14(1): 24508 doi: 10.1038/s41598-024-76106-5[2] Meng F L, Li M W, Zhang R Z, et al. Room temperature n-butanol detection by Ag-modified In2O3 gas sensor with UV excitation. Ceram Int, 2025, 51(2): 1764 doi: 10.1016/j.ceramint.2024.11.152[3] Liao Z J, Yuan Z Y, Gao H L, et al. Novel Co3O4-CuO-CuOHF porous sheet for high sensitivity n-butanol gas sensor at low temperature. Sens Actuat B Chem, 2023, 384: 133619 doi: 10.1016/j.snb.2023.133619[4] Najafi P, Ghaemi A. Chemiresistor gas sensors: Design, challenges, and strategies: A comprehensive review. Chem Eng J, 2024, 498: 154999 doi: 10.1016/j.cej.2024.154999[5] Thayil R, Parne S R. Nanostructured zinc oxide and selenide-based materials for gas sensing application: review. J Mater Sci Mater Electron, 2025, 36(5): 322 doi: 10.1007/s10854-025-14401-1[6] Li Y, Shan L X, Wang R C, et al. Enhanced n-butanol sensing performance of SnO2/ZnO nanoflowers fabricated via a facile solvothermal method. Ceram Int, 2022, 48(15): 22426 doi: 10.1016/j.ceramint.2022.04.256[7] Santos G S M, de Sá B S, Perfecto T M, et al. MOF-derived Co3O4-ZnO heterostructure for 3-methyl-1-butanol detection. Sens Actuat B Chem, 2024, 408: 135533 doi: 10.1016/j.snb.2024.135533[8] Lei Q, Li H R, Zhang H, et al. Three-dimensional hierarchical CuO gas sensor modified by Au nanoparticles. J Semicond, 2019, 40(2): 022101 doi: 10.1088/1674-4926/40/2/022101[9] Thakur N, Murthy H, Arumugam S, et al. Direct ink writing of nickel oxide-based thin films for room temperature gas detection. J Semicond, 2025, 46(1): 012606 doi: 10.1088/1674-4926/24080025[10] Kwoka M, Kulis-Kapuscinska A, Zappa D, et al. Novel insight on the local surface properties of ZnO nanowires. Nanotechnology, 2020, 31(46): 465705 doi: 10.1088/1361-6528/ab8dec[11] Yue Q, Liu T, Mu Y, et al. Highly responsive and swift recovery triethylamine gas sensor based on NiCo2O4-ZnO p-n heterojunction. Sens Actuat B Chem, 2024, 410: 135666 doi: 10.1016/j.snb.2024.135666[12] Wei X S, Yang X C, Guo Y, et al. UV-enhanced Mg: MOF-ZnO sensor for n-butanol gas detection at a lower operating temperature. Appl Surf Sci, 2024, 678: 161138 doi: 10.1016/j.apsusc.2024.161138[13] Xian J B, Li J, Wang W J, et al. Enhanced specific surface area of ZIF-8 derived ZnO induced by sulfuric acid modification for high-performance acetone gas sensor. Appl Surf Sci, 2023, 614: 156175 doi: 10.1016/j.apsusc.2022.156175[14] Kim S J, Lee J, Bae J S, et al. The impact of ZIF-8 particle size control on low-humidity sensor performance. Nanomaterials, 2024, 14(3): 284 doi: 10.3390/nano14030284[15] Yi M, Li H R, Huang D D, et al. ZIF-8-derived ZnO doped with in for high-performance ethanol gas sensor. J Mater Sci Mater Electron, 2024, 35(5): 342 doi: 10.1007/s10854-024-12062-0[16] Cai H, Luo H, Hu F R, et al. High-response n-butanol gas sensor based on quasi-Zn-MOFs with tunable surface oxygen vacancies. J Alloys Compd, 2025, 1010: 177274 doi: 10.1016/j.jallcom.2024.177274[17] Wan G X, Xue R J, Qin T, et al. Acetone detection at low temperature using a gas nano-sensor based on ZnO flakes loaded with metal organic-skeleton ZIF-8. Mater Today Commun, 2023, 37: 107561 doi: 10.1016/j.mtcomm.2023.107561[18] Zhou J M, Wang X D, Zhang Y P, et al. Rare earth element Ce-doped floral In2O3 with high sensing performance for n-butanol. Vacuum, 2025, 231: 113761 doi: 10.1016/j.vacuum.2024.113761[19] Suryawanshi V N, Varpe A S, Deshpande M D. The influence of rare earth (RE) dopants on structural, optical and gas sensing properties of spray deposited PbO thin films, where RE = Ce, Nd and Eu. Pramana, 2022, 96(1): 38 doi: 10.1007/s12043-021-02280-0[20] Sayago I, Santos J P, Sánchez-Vicente C. The effect of rare earths on the response of photo UV-activate ZnO gas sensors. Sensors, 2022, 22(21): 8150 doi: 10.3390/s22218150[21] Zhao S K, Shen Y B, Li A, et al. Effects of rare earth elements doping on gas sensing properties of ZnO nanowires. Ceram Int, 2021, 47(17): 24218 doi: 10.1016/j.ceramint.2021.05.133[22] Yu S G, Zhang H Y, Lin C C, et al. The enhancement of humidity sensing performance based on Eu-doped ZnO. Curr Appl Phys, 2019, 19(2): 82 doi: 10.1016/j.cap.2018.11.015[23] Sun X Y, Tang M X, Yu M Q, et al. UV-activated CH4 gas sensor based on Pd@Ni/ZnO microspheres. Mater Today Commun, 2024, 40: 109551 doi: 10.1016/j.mtcomm.2024.109551[24] Liu H, Liu J L, Liu Y C, et al. UV light-activated Eu/ZnO flower-like microsphere for detecting NO2 gas with high response. Ceram Int, 2024, 50(20): 39654 doi: 10.1016/j.ceramint.2024.07.345[25] Sun X Y, Zhang Y, Wang Y H, et al. UV-activated AuAg/ZnO microspheres for high-performance methane sensor at room temperature. Ceram Int, 2024, 50(17): 30552 doi: 10.1016/j.ceramint.2024.05.352[26] Wei X S, Yang X C, Guo Y, et al. Room temperature detection of n-butanol Ce-doped MOF: ZnO sensor under UV activation. Ceram Int, 2024, 50(21): 41943 doi: 10.1016/j.ceramint.2024.08.192[27] Cravillon J, Nayuk R, Springer S, et al. Controlling zeolitic imidazolate framework nano- and microcrystal formation: Insight into crystal growth by time-resolved In situ static light scattering. Chem Mater, 2011, 23(8): 2130 doi: 10.1021/cm103571y[28] Thayil R, Parne S R. Tuning MoS2 nanostructures for superior room-temperature toluene sensing. Talanta Open, 2025, 11: 100402 doi: 10.1016/j.talo.2025.100402[29] Nair M G, Nirmala M, Rekha K, et al. Structural, optical, photo catalytic and antibacterial activity of ZnO and Co doped ZnO nanoparticles. Mater Lett, 2011, 65(12): 1797 doi: 10.1016/j.matlet.2011.03.079[30] Umar A, Akbar S, Kumar R, et al. Ce-doped ZnO nanostructures: A promising platform for NO2 gas sensing. Chemosphere, 2024, 349: 140838 doi: 10.1016/j.chemosphere.2023.140838[31] Hastir A, Kohli N, Singh R C. Comparative study on gas sensing properties of rare earth (Tb, Dy and Er) doped ZnO sensor. J Phys Chem Solids, 2017, 105: 23 doi: 10.1016/j.jpcs.2017.02.004[32] Pan S S, Guo Y, Chen G, et al. MOFs-derived synthesis of Ni-doped ZnO nanostructutred material towards excellent N-butanol sensing performance and long-term stability. J Mater Sci Mater Electron, 2022, 33(10): 7501 doi: 10.1007/s10854-022-07888-5[33] Wang Y, Meng X N, Cao J L. Rapid detection of low concentration CO using Pt-loaded ZnO nanosheets. J Hazard Mater, 2020, 381: 120944 doi: 10.1016/j.jhazmat.2019.120944[34] Sun K, Zhan G, Zhang L, et al. Highly sensitive NO2 gas sensor based on ZnO nanoarray modulated by oxygen vacancy with Ce doping. Sens Actuat B Chem, 2023, 379: 133294 doi: 10.1016/j.snb.2023.133294[35] Mokoena T P, Swart H C, Hillie K T, et al. Engineering of rare-earth Eu3+ ions doping on p-type NiO for selective detection of toluene gas sensing and luminescence properties. Sens Actuat B Chem, 2021, 347: 130530 doi: 10.1016/j.snb.2021.130530[36] Dong Q, Su H L, Xu J Q, et al. Influence of hierarchical nanostructures on the gas sensing properties of SnO2 biomorphic films. Sens Actuat B Chem, 2007, 123(1): 420 doi: 10.1016/j.snb.2006.09.018[37] Pan Z Z, Sun F Q, Zhu X M, et al. Electrodeposition-based in situ construction of a ZnO-ordered macroporous film gas sensor with enhanced sensitivity. J Mater Chem A, 2019, 7(3): 1287 doi: 10.1039/C8TA09920K[38] Vargas W E, Niklasson G A. Applicability conditions of the kubelka-munk theory. Appl Opt, 1997, 36(22): 5580 doi: 10.1364/AO.36.005580[39] Zhang L, Kang Y L, Tang Y, et al. UV-Activated ZnO−NiO heterojunction sensor for ethanol gas detection at low working temperature. Mater Sci Semicond Process, 2024, 169: 107925 doi: 10.1016/j.mssp.2023.107925[40] Afsharmanesh E, Haratizadeh H. Improving acetone gas selectivity and UV sensing inSnO2/ZnO nanostructured sensors by changing its compositions. Phys Scr, 2025, 100(6): 065954 doi: 10.1088/1402-4896/add222[41] Kundu S, Sain S, Satpati B, et al. Structural interpretation, growth mechanism and optical properties of ZnO nanorods synthesized by a simple wet chemical route. RSC Adv, 2015, 5(29): 23101 doi: 10.1039/C5RA01152C[42] Hjiri M, Bahanan F, Aida M S, et al. High performance CO gas sensor based on ZnO nanoparticles. J Inorg Organomet Polym Mater, 2020, 30(10): 4063 doi: 10.1007/s10904-020-01553-2[43] Li M, Chang J Q, Deng Z H, et al. Discriminating gas molecules at room temperature by UV light modulation (ULM) of nonselective metal oxide sensors. Sens Actuat B Chem, 2023, 378: 133115 doi: 10.1016/j.snb.2022.133115[44] Sun Y Q, Li J A, He D W, et al. Recent progress on performances and mechanisms of carbon dots for gas sensing. Luminescence, 2023, 38(7): 896 doi: 10.1002/bio.4306[45] Selvaraj B, Balaguru Rayappan J B, Jayanth Babu K. Influence of calcination temperature on the growth of electrospun multi-junction ZnO nanowires: A room temperature ammonia sensor. Mater Sci Semicond Process, 2020, 112: 105006 doi: 10.1016/j.mssp.2020.105006[46] Duan P Y, Wang H W, Peng Q K, et al. Ultra-effective room temperature gas discrimination based on monolithic Pd@MOF-derived porous nanocomposites: An exclusive scheme with photoexcitation. J Mater Chem A, 2024, 12(7): 3896 doi: 10.1039/D3TA05740B[47] Zhang S Q, Ling W Y, Zhao T Y, et al. High response ZnO gas sensor derived from Tb@Zn-MOFs to acetic acid under UV excitation. Sens Actuat A Phys, 2024, 365: 114862 doi: 10.1016/j.sna.2023.114862[48] Hjiri M, Alkaoud A, Neri G. Low operating temperature of UV photo-activated in-doped ZnO NO2 sensors. Appl Phys A, 2023, 129(12): 861 doi: 10.1007/s00339-023-07137-4[49] Wang Q, Hong J S, Zhang Z L, et al. Visible light-activated ethanol sensor based on flower-like N3-loaded ZnO composites. Sens Actuat B Chem, 2022, 370: 132399 doi: 10.1016/j.snb.2022.132399[50] Qu Y W, Zhang J. Bimetallic Co−Mn catalysts for synergistic enhancement of VOC gas-sensing performance of ZnO hierarchical nanostructures. RSC Adv, 2024, 14(15): 10358 doi: 10.1039/D4RA00553H[51] Guo S Y, Chen X D, Chen H, et al. Ultrasensitive room temperature formaldehyde sensors based on F doped ZnO nanostructures activated by UV light. Langmuir, 2024, 40(46): 24592 doi: 10.1021/acs.langmuir.4c03304[52] Tang J F, Fang C C, Hsu C L. Enhanced organic gas sensor based on Cerium- and Au-doped ZnO nanowires via low temperature one-pot synthesis. Appl Surf Sci, 2023, 613: 156094 doi: 10.1016/j.apsusc.2022.156094 -
Proportional views





 Yinzhong Liu is a master candidate at Shanghai University, Shanghai, China. Her research interest is the preparation and application of REE-doped gas sensing materials.
Yinzhong Liu is a master candidate at Shanghai University, Shanghai, China. Her research interest is the preparation and application of REE-doped gas sensing materials. Xuechun Yang is affiliated with the Key Laboratory of Organic Compound Pollution Control Engineering at Shanghai University, Shanghai, China. She earned her Ph.D. degree in Materials Physics and Chemistry from the Shanghai University. Her work mainly includes the preparation of inorganic/organic nanocomposites and the manufacture of chemical sensors.
Xuechun Yang is affiliated with the Key Laboratory of Organic Compound Pollution Control Engineering at Shanghai University, Shanghai, China. She earned her Ph.D. degree in Materials Physics and Chemistry from the Shanghai University. Her work mainly includes the preparation of inorganic/organic nanocomposites and the manufacture of chemical sensors. Yun Guo is currently associate professor working at Shanghai University, Shanghai, China. She earned her B.S. degree in Mineralogy from Nanjing University and Ph.D. degree in Materials Science from Shanghai University. Her current research interests focus on the preparation and performance of semiconductor gas sensing materials and devices.
Yun Guo is currently associate professor working at Shanghai University, Shanghai, China. She earned her B.S. degree in Mineralogy from Nanjing University and Ph.D. degree in Materials Science from Shanghai University. Her current research interests focus on the preparation and performance of semiconductor gas sensing materials and devices.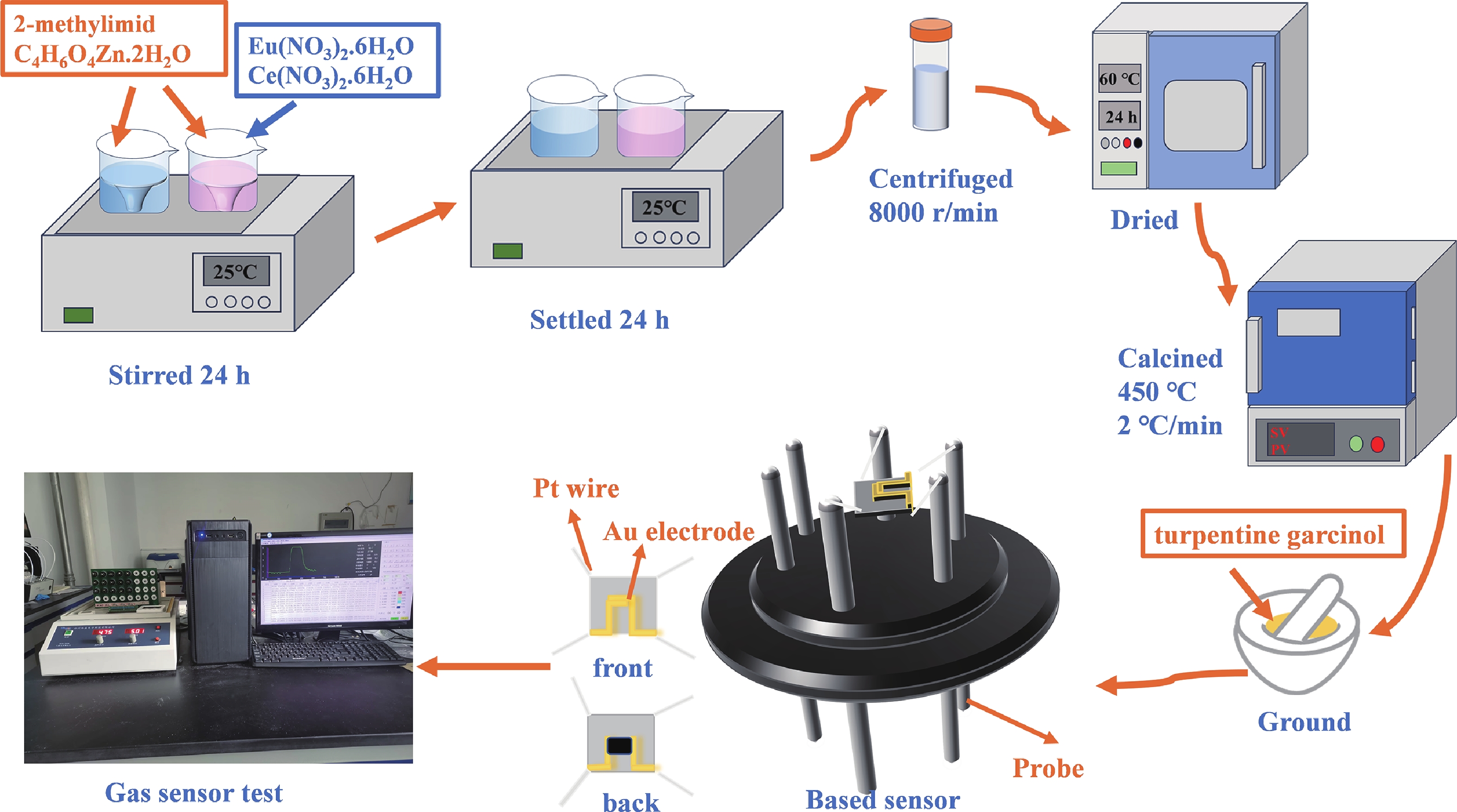
 DownLoad:
DownLoad: