| Citation: |
Yun Gui, Xu Zhang, Yuan Wang, Ming Liu, Weihua Pei, Kai Liang, Suibiao Huang, Bin Li, Hongda Chen. A multi-channel fully differential programmable integrated circuit for neural recording application[J]. Journal of Semiconductors, 2013, 34(10): 105009. doi: 10.1088/1674-4926/34/10/105009
****
Y Gui, X Zhang, Y Wang, M Liu, W H Pei, K Liang, S B Huang, B Li, H D Chen. A multi-channel fully differential programmable integrated circuit for neural recording application[J]. J. Semicond., 2013, 34(10): 105009. doi: 10.1088/1674-4926/34/10/105009.
|
A multi-channel fully differential programmable integrated circuit for neural recording application
DOI: 10.1088/1674-4926/34/10/105009
More Information
-
Abstract
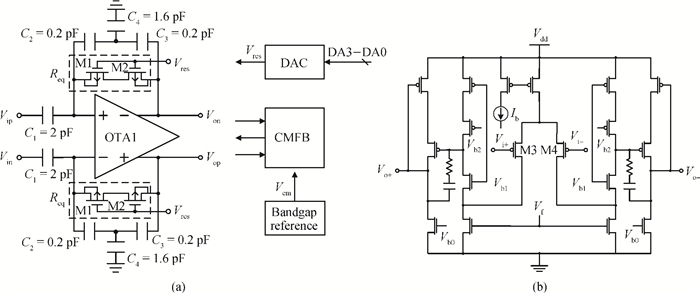
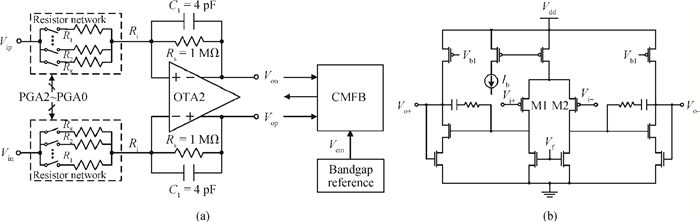
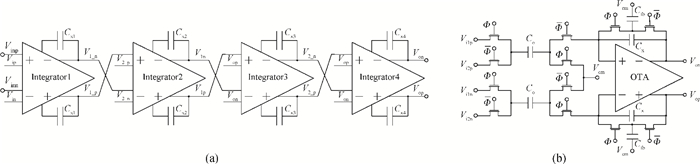
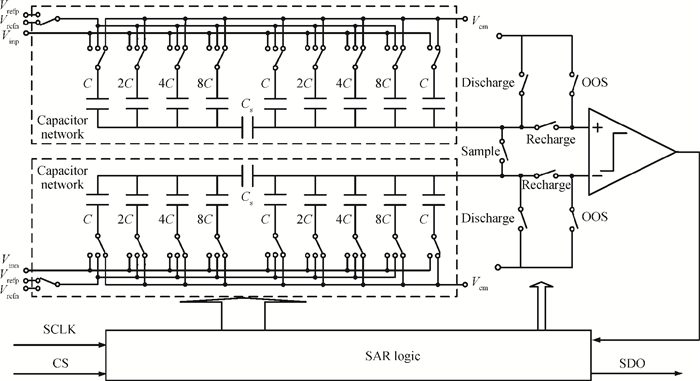
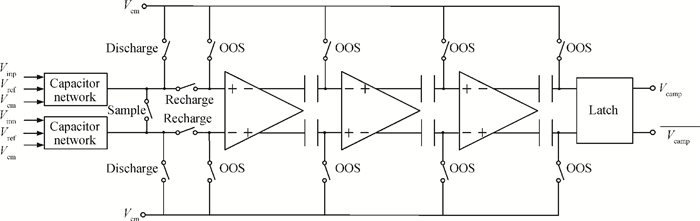
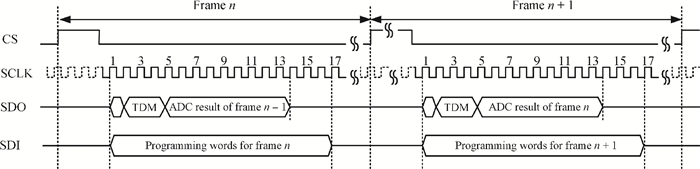
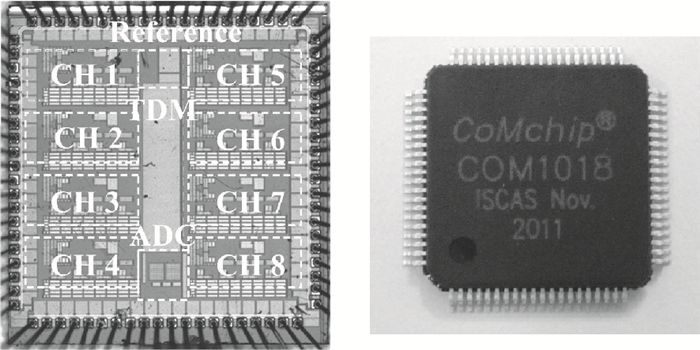
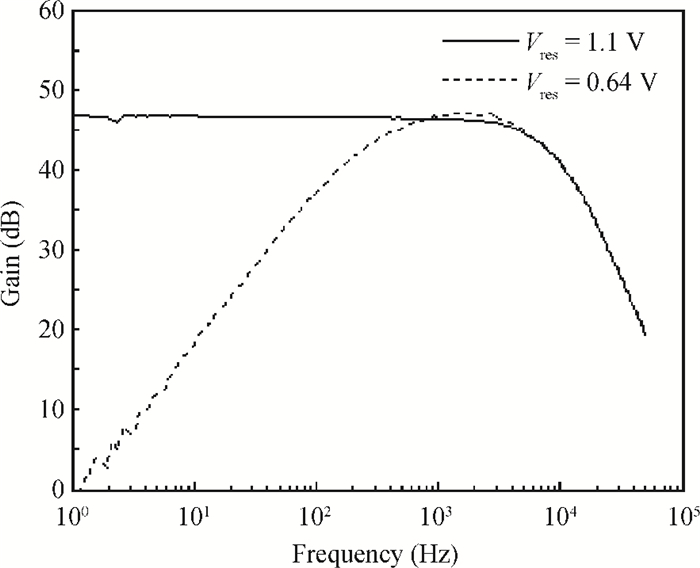
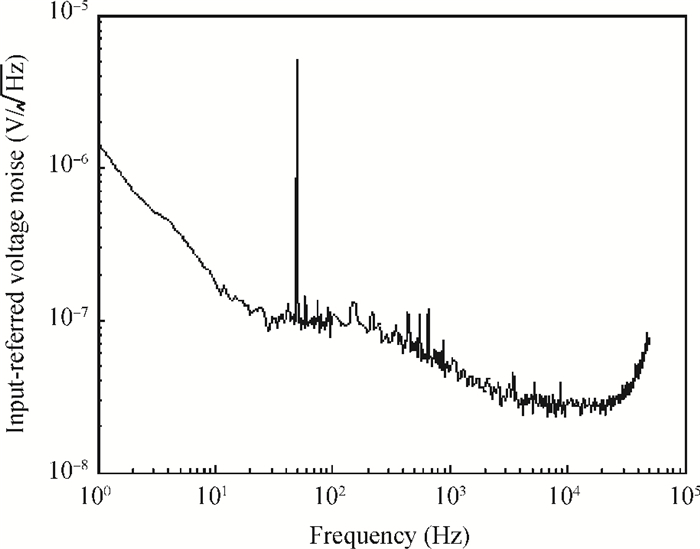
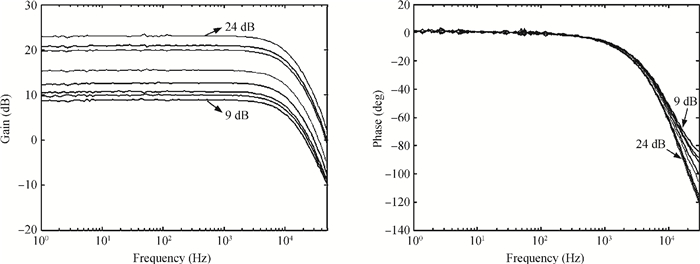
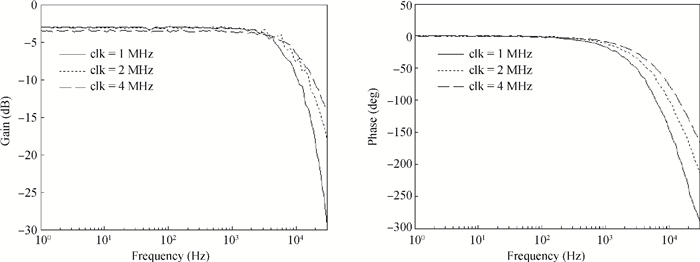
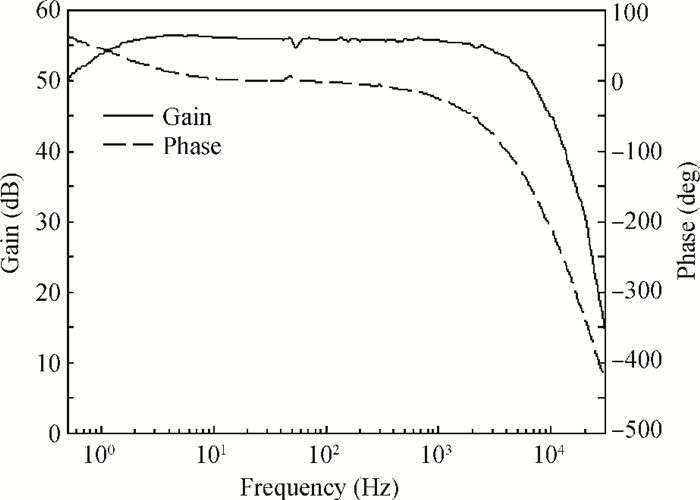
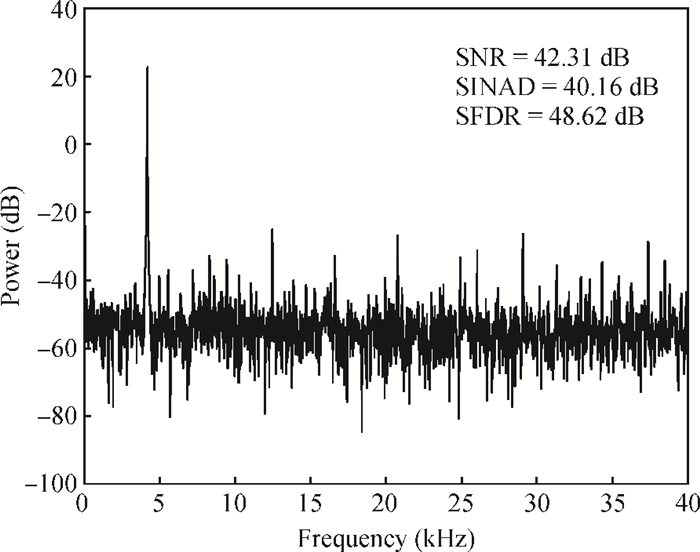
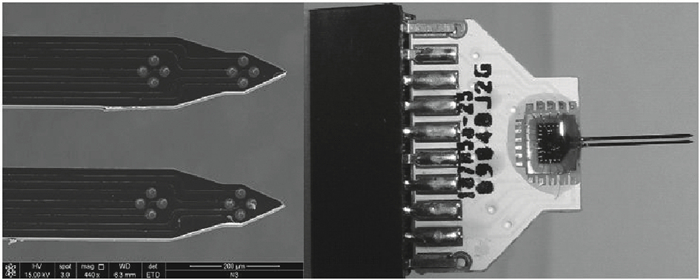
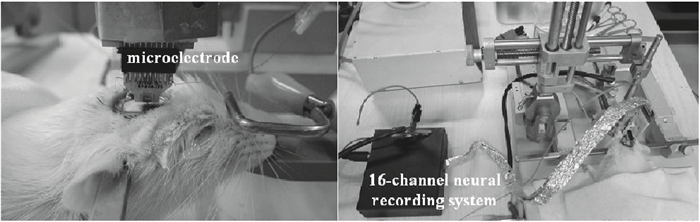
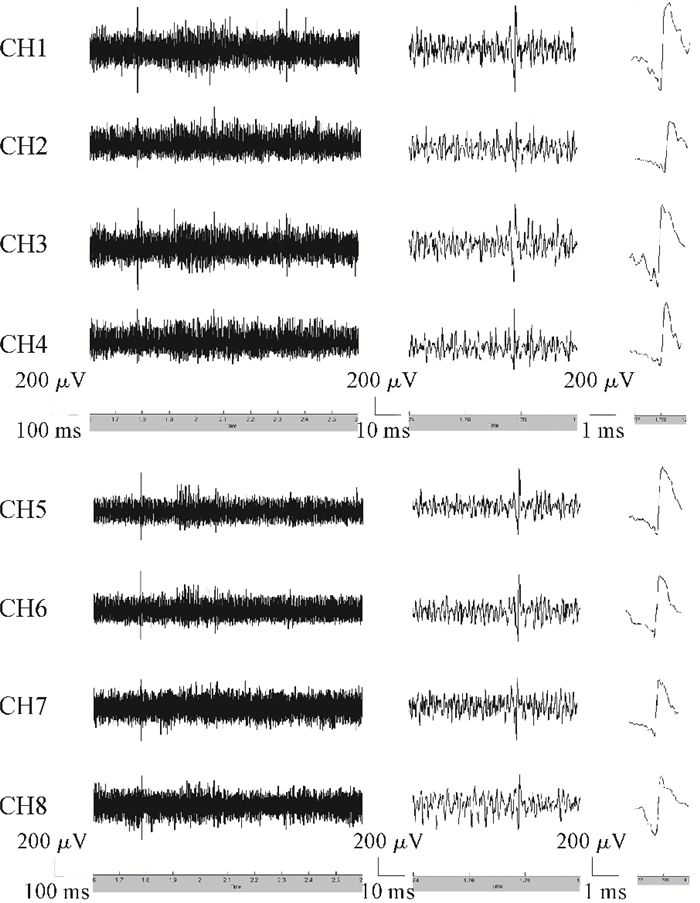
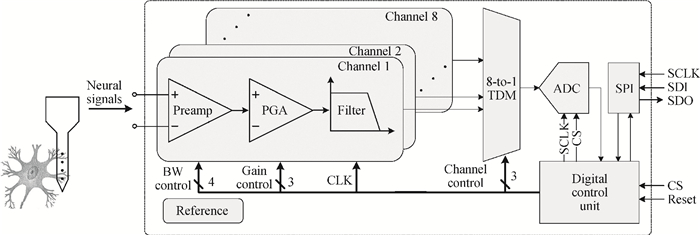
A multi-channel, fully differential programmable chip for neural recording application is presented. The integrated circuit incorporates eight neural recording amplifiers with tunable bandwidth and gain, eight 4th-order Bessel switch capacitor filters, an 8-to-1 analog time-division multiplexer, a fully differential successive approximation register analog-to-digital converter (SAR ADC), and a serial peripheral interface for communication. The neural recording amplifier presents a programmable gain from 53 dB to 68 dB, a tunable low cut-off frequency from 0.1 Hz to 300 Hz, and 3.77 μVrms input-referred noise over a 5 kHz bandwidth. The SAR ADC digitizes signals at maximum sampling rate of 20 kS/s per channel and achieves an ENOB of 7.4. The integrated circuit is designed and fabricated in 0.18-μm CMOS mix-signal process. We successfully performed a multi-channel in-vivo recording experiment from a rat cortex using the neural recording chip. -
References
[1] Mountcastle V B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol, 1957, 20(4):408 http://jn.physiology.org/content/20/4/408[2] Hubel D H, Wiesel T N. Receptive fields of single neurons in the cat's striate cortex. J Physiol, 1959, 148(3):574 doi: 10.1113/jphysiol.1959.sp006308[3] Pei W, Zhu L, Wang S, et al. Multi-channel micro neural probe fabricated with SOI. Sci China Tech Sci, 2012, 52(5):1187 doi: 10.1007/s11431-008-0272-9?no-access=true[4] Zhao H, Pei W, Chen S, et al. A composite insulation structure for silicon-based planar neuroprobes. Sci China Tech Sci, 2012, 55(9):2436 doi: 10.1007/s11431-012-4856-z[5] Rousche P J, Pellinen D S, Pivin D P, et al. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans Biomed Eng, 2001, 48(3):361 doi: 10.1109/10.914800[6] Li X, Pei W, Tang R, et al. Investigation of flexible electrodes modified by TiN, Pt black and IrOx. Sci China Tech Sci, 2011, 54(9):2305 doi: 10.1007/s11431-011-4436-7[7] Aziz J, Abdelhalim K, Shulyzki R, et al. 256-channel neural recording and delta compression microsystem with 3D electrodes. IEEE J Solid-State Circ, 2009, 44(3):995 doi: 10.1109/JSSC.2008.2010997[8] Harrison R R, Kier R J, Chestek C A, et al. Wireless neural recording with single low-power integrated circuit. IEEE Trans Neural Syst Rehabil Eng, 2009, 17(4):322 doi: 10.1109/TNSRE.2009.2023298[9] Sodagar A, Wise K D, Najafi K. A wireless implantable microsystem for multichannel neural recording. IEEE Trans Microwave Theory Tech, 2009, 57(10):2565 doi: 10.1109/TMTT.2009.2029957[10] Dumortier C, Gosselin B, Sawan M. Wavelet transforms dedicated to compress recorded ENGs from multichannel implants:comparative architectural study. Proc of IEEE Int Symp Circ Syst, 2006:2129 http://ieeexplore.ieee.org/document/1693038/?reload=true&tp=&arnumber=1693038&openedRefinements%3D*%26filter%3DAND(AND(AND(NOT(4283010803)),AND(NOT(4283010803))),AND(NOT(4283010803)))%26pageNumber%3D11%26rowsPerPage%3D100%26queryText%3D(real%20discrete%20wavelet%20transform%20%20rdwt%20de)[11] Horiuchi T, Swindell T, Sander D, et al. A low-power CMOS neural amplifier with amplitude measurements for spike sorting. Proc of IEEE Int Symp Circ Syst, 2004, 4:29 http://ieeexplore.ieee.org/document/1328932/[12] O'Driscoll S, Meng T H, Shenoy K V, et al. Neurons to silicon:implantable prosthesis processor. Proc of IEEE Int Solid-State Circuits Conf, 2006:2248 https://www.infona.pl/resource/bwmeta1.element.ieee-art-000001696287[13] Harrison R R, Charles C. A low-power low-noise CMOS amplifier for neural recording applications. IEEE J Solid-State Circuits, 2003, 38(6):958 doi: 10.1109/JSSC.2003.811979[14] Chae M S, Liu W, Sivaprakasam M. Design optimization for integrated neural recording systems. IEEE J Solid-State Circuits, 2008, 43(9):1931 doi: 10.1109/JSSC.2008.2001877[15] Zhang X. Studies on application specific integrated circuits for neural interfaces. Dissertation for the Doctoral Degree, Beijing:Graduate School of Chinese Academy of Sciences, 2010, 70[16] Zhang X, Pei W, Huang B, et al. A low-noise fully-differential CMOS preamplifier for neural recording applications. Sci China Inf Sci, 2012, 55(2):441 doi: 10.1007/s11432-011-4333-5[17] Olsson R Ⅲ, Buhl D, Sirota A, et al. Band-tunable and multiplexed integrated circuits for simultaneous recording and stimulation with microelectrode arrays. IEEE Trans Biomed Eng, 2005, 52(7):1303 doi: 10.1109/TBME.2005.847540[18] Guillory K S, Normann R A, A 100-channel system for real time detection and storage of extracellular spike waveforms. J Neurosci Methods, 1999, 91(1):21 http://www.sciencedirect.com/science/article/pii/S016502709900076X?via%3Dihub[19] Culurciello E, Andreou A. An 8-bit, 1 mW successive approximation ADC in SOI CMOS. Proc of IEEE Int Symp Circ Syst, 2003, 1:301 http://ieeexplore.ieee.org/document/1205560/authors[20] Gosselin B, Ayoub A E, Roy J, et al. A mixed-signal multichip neural recording interface with bandwidth reduction. IEEE Trans Biomed Circ and Syst, 2009, 3(3):129 doi: 10.1109/TBCAS.2009.2013718[21] Kmon P, Zoladz M, Grybos P, et al. Design and measurements of 64-channel ASIC for neural signal recording. Proc of 31st Int Conf IEEE Eng Med Biol Soc, 2009:528 http://ieeexplore.ieee.org/document/5333629/[22] Shahrokhi F, Abdelhalim K, Serletis D, et al. The 128-channel fully differential digital integrated neural recording and stimulation interface. IEEE Trans Biomed Circ and Syst, 2010, 4(3):149 doi: 10.1109/TBCAS.2010.2041350 -
Proportional views





 DownLoad:
DownLoad: