| Citation: |
Qiang Zhao, Maoxiu Zhou, Wei Zhang, Qi Liu, Xiaofeng Li, Ming Liu, Yuehua Dai. Effects of interaction between defects on the uniformity of doping HfO2-based RRAM:a first principle study[J]. Journal of Semiconductors, 2013, 34(3): 032001. doi: 10.1088/1674-4926/34/3/032001
****
Q Zhao, M X Zhou, W Zhang, Q Liu, X F Li, M Liu, Y H Dai. Effects of interaction between defects on the uniformity of doping HfO2-based RRAM:a first principle study[J]. J. Semicond., 2013, 34(3): 032001. doi: 10.1088/1674-4926/34/3/032001.
|
Effects of interaction between defects on the uniformity of doping HfO2-based RRAM:a first principle study
DOI: 10.1088/1674-4926/34/3/032001
More Information
-
Abstract
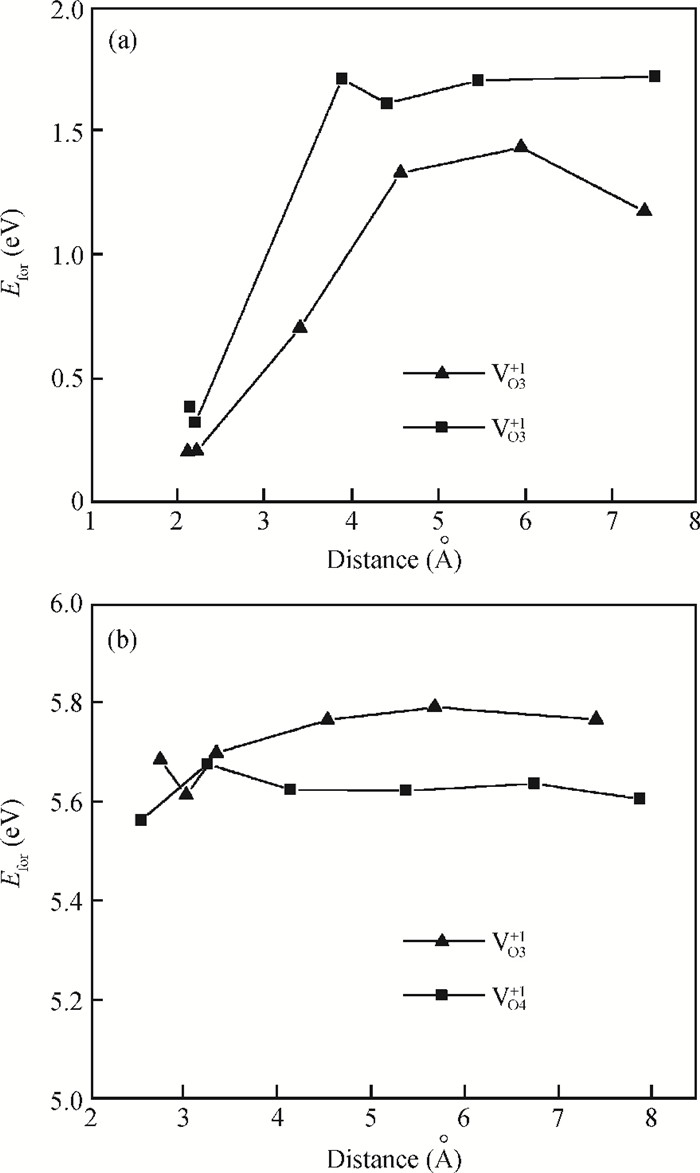
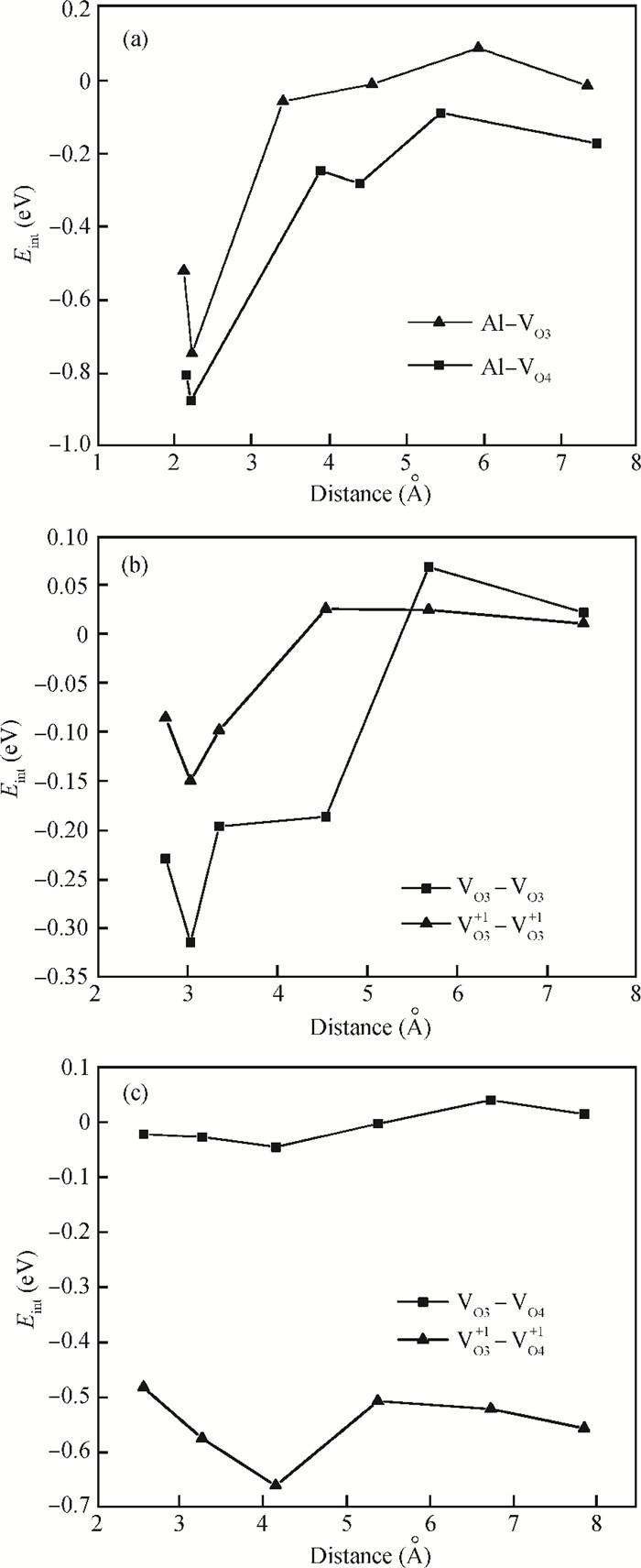
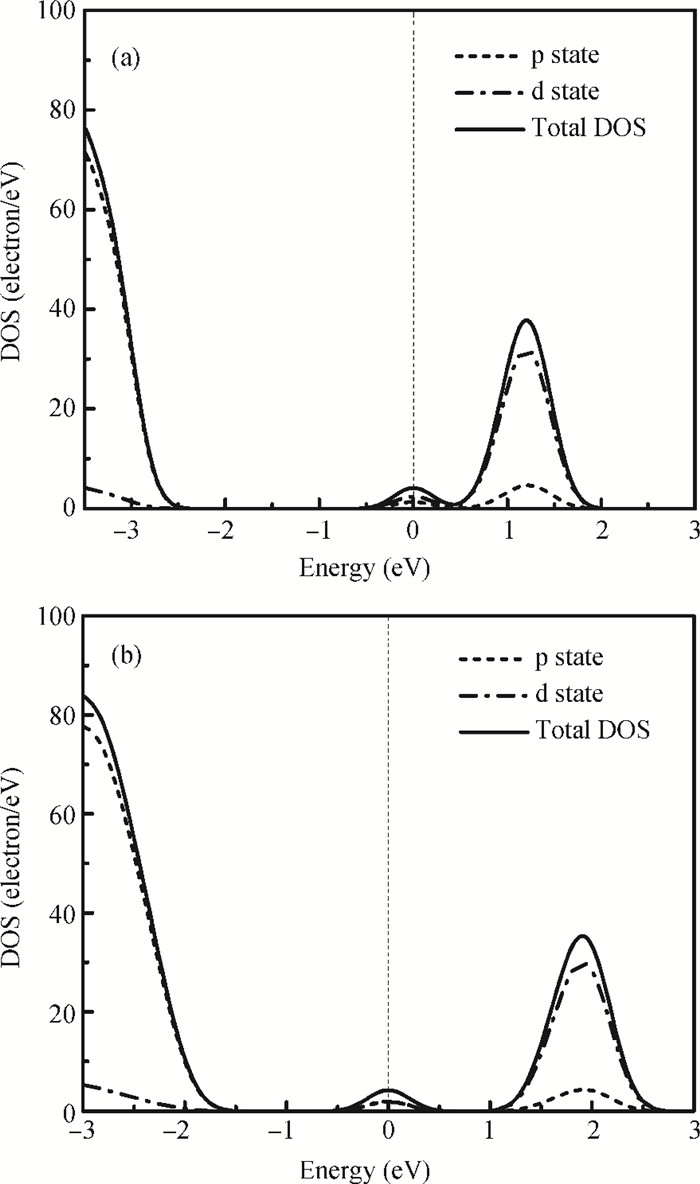
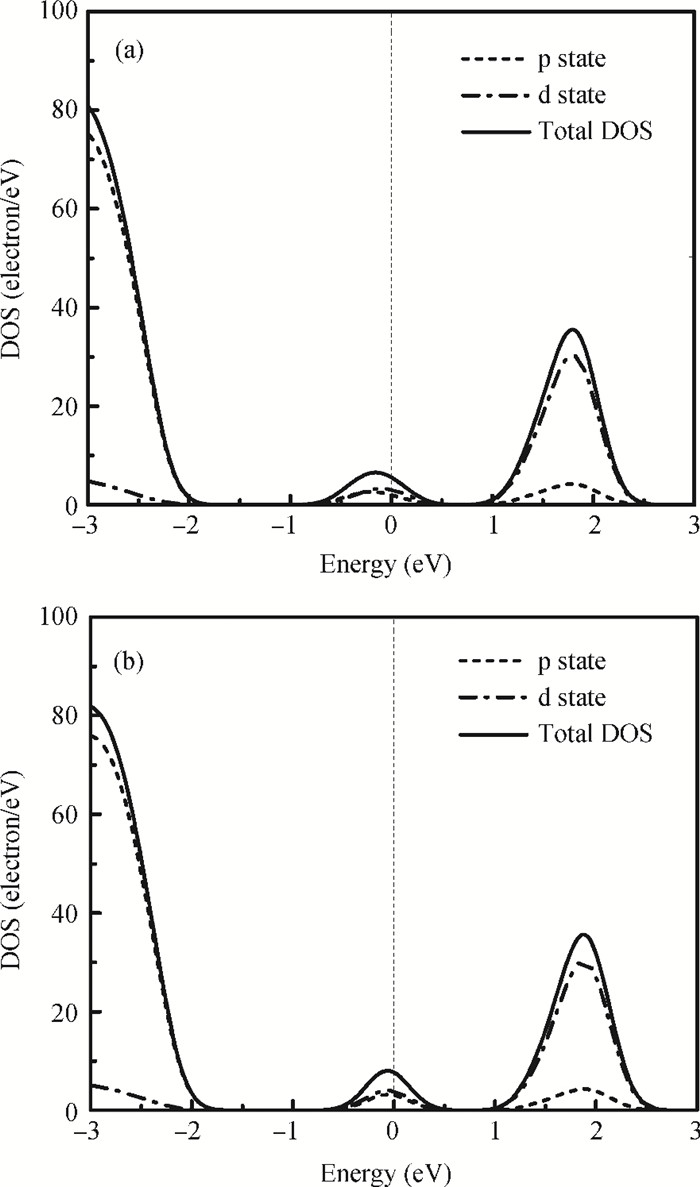
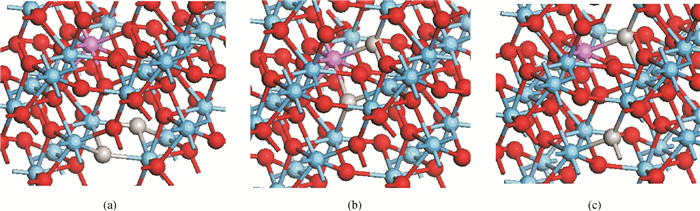
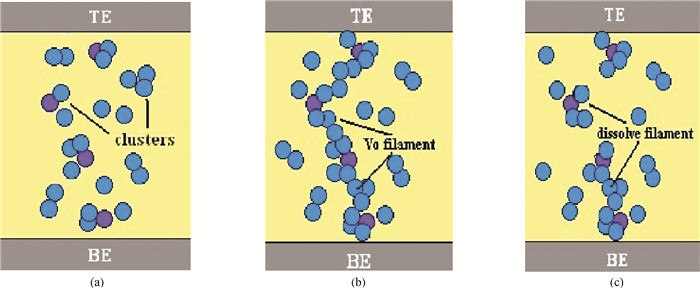
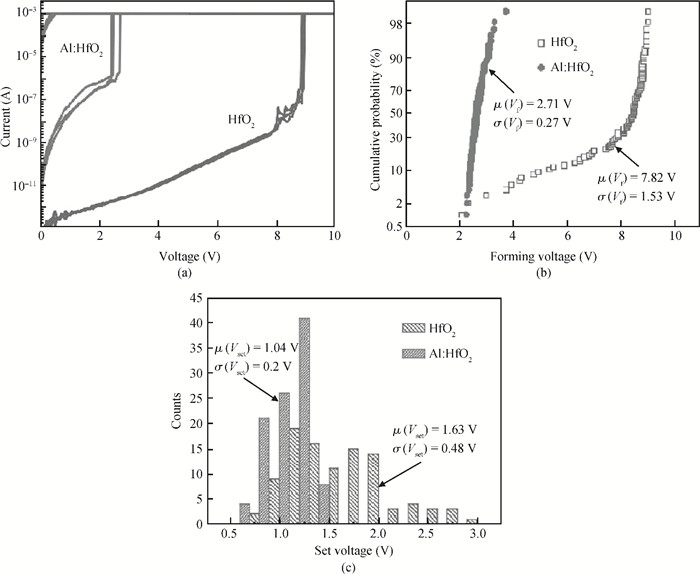
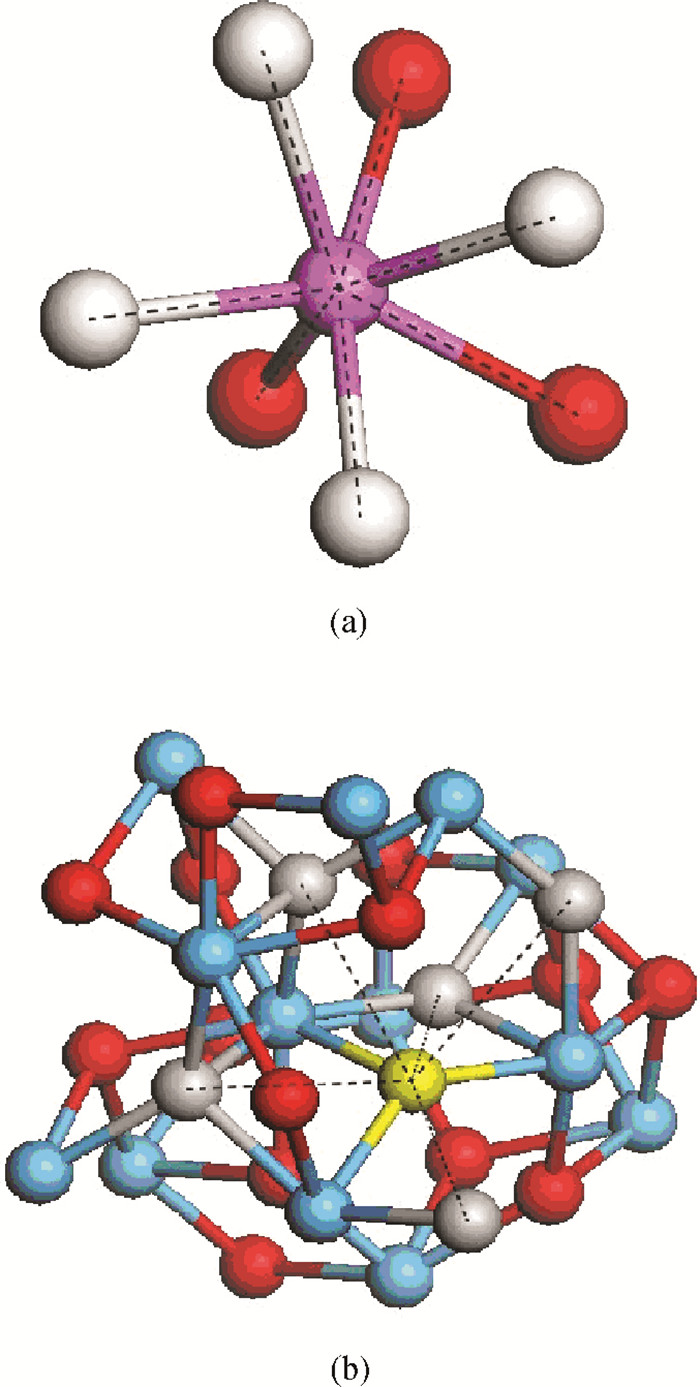
The physical mechanism of doping effects on switching uniformity and operation voltage in Al-doped HfO2 resistive random access memory (RRAM) devices is proposed from another perspective:defects interactions, based on first principle calculations. In doped HfO2, dopant is proved to have a localized effect on the formation of defects and the interactions between them. In addition, both effects cause oxygen vacancies (VO) to have a tendency to form clusters and these clusters are easy to form around the dopant. It is proved that this process can improve the performance of material through projected density of states (PDOS) analysis. For VO filament-type RRAM devices, these clusters are concluded to be helpful for the controllability of the switching process in which oxygen vacancy filaments form and break. Therefore, improved uniformity and operation voltage of Al-doped HfO2 RRAM devices is achieved.-
Keywords:
- RRAM,
- hafnium oxide,
- localized effect,
- oxygen vacancy,
- DFT
-
References
[1] Yang Y, Pan F, Liu Q, et al. Fully room-temperature-fabricated nonvolatile resistive memory for ultrafast and high-density memory application. Nano Lett, 2009, 9(4):1636 doi: 10.1021/nl900006g[2] Waser R, Aono M. Nanoionics based resistive switching memories. Nature Mater, 2007, 6(11):833 doi: 10.1038/nmat2023[3] Zhou M X, Zhao Q, Zhang W, et al. The conductive path in HfO2:first principle study. Journal of Semiconductors, 2012, 33(7):072002 doi: 10.1088/1674-4926/33/7/072002[4] Li Y T, Wang Y, Liu S, et al. Improvement of resistive switching uniformity in TiOx films by nitrogen annealing. J Korean Phys Soc, 2011, 58(3):L407[5] Lv H, Wan H, Tang T. Improvement of resistive switching uniformity by introducing a thin GST interface layer. IEEE Electron Device Lett, 2010, 31(9):978 doi: 10.1109/LED.2010.2055534[6] Liu Q, Long S, Lv H, et al. Controllable growth of nanoscale conductive filaments in solid electrolyte based ReRAM by using a metal nanocrystal covered bottom electrode. ACSnano, 2010, 4(10):6162[7] Park J, Jo M, Lee J, et al. Improved switching uniformity and speed in filament type RRAM using lighting rod effect. IEEE Electron Device Lett, 2011, 32(1):63 doi: 10.1109/LED.2010.2084560[8] Gao B, Zhang H, Yu S, et al. Oxide based RRAM:uniformity improvement using a new material oriented methodology. Symp on VLSI Technol, 2009:30 http://ieeexplore.ieee.org/document/5200623/[9] Zhang H W, Liu L F, Gao B, et al. Gd doping effect on performance of HfO2 based resistive switching memory devices using implantation approach. Appl Phys Lett, 2011, 98:042105 doi: 10.1063/1.3543837[10] Foster A S, Lopez Gejo F, Shluger A L, et al. Vacancy and interstitial defects in hafnia. Phys Rev B, 2002, 65:174117 doi: 10.1103/PhysRevB.65.174117[11] Hou Z F, Gong X G, Li Q. Al-induced reduction of the oxygen diffusion in HfO2:an ab initio study. J Phys:Condense Matter, 2008, 20:135206 doi: 10.1088/0953-8984/20/13/135206[12] Umezawa N, Sato M, Shiraishi M. Reduction in charged defects associated with oxygen vacancies in hafnia by magnesium incorporation:first-principles study. Appl Phys Lett, 2008, 93:223104 doi: 10.1063/1.3040306[13] Zhang H, Gao B, Sun B, et al. Ionic doping effect in ZrO2 resistive switching memory. Appl Phys Lett, 2010, 96:123502 doi: 10.1063/1.3364130[14] Nadimi E, Ottking R, Plänitz P, et al. Interaction of oxygen vacancies and lanthanum in Hf-based high-k dielectrics:an ab initio investigation. J Phys Condens Matter, 2011, 23:365502 doi: 10.1088/0953-8984/23/36/365502[15] Park S, Ahn H S, Lee C K, et al. Interaction and ordering of vacancy defects in NiO. Phys Rev B, 2008, 77:134103 doi: 10.1103/PhysRevB.77.134103[16] He L, Liao Z, Wu H, et al. Memory and threshold resistance switching in Ni/NiO core-shell nanowires. Nanoletters, 2011, 11(11):4601 doi: 10.1021/nl202017k[17] Segall M D, Lindan P J D, Probert M J, et al. First principles simulation:ideas, illustrations and the CASTEP code. J Phys Condens Matter, 2002, 14:2717 doi: 10.1088/0953-8984/14/11/301[18] Li Quan, Koo K M, Lau W M, et al. Effects of Al addition on the native defects in hafnia. Appl Phys Lett, 2006, 88:182903 doi: 10.1063/1.2196470[19] Van de Walle C G, Neugebauer J. First principles calculations for defects and impurities:applications to Ⅲ-nitrides. J Appl Phys, 2004, 95:3851 doi: 10.1063/1.1682673[20] Peng C, Chang W, Lee Y, et al. Electrochem improvement of resistive switching stability of HfO2 films with Al doping by atomic layer deposition. Solid-State Lett, 2012, 15(4):H88 http://esl.ecsdl.org/content/15/4/H88.full.pdf[21] Nakayama M, Martin M. First-principles study on defect chemistry and migration of oxide ions in ceria doped with rare-earth cations. Phys Chem Chem Phys, 2009, 11:3241 doi: 10.1039/b900162j[22] Kamiya K, Yang M Y, Park S G, et al. On-off switching mechanism of resistive random access memories based on the formation and disruption of oxygen vacancy conduction channels. Appl Phys Lett, 2012, 100:073502 doi: 10.1063/1.3685222[23] Finocchi F, Goniakowski J, Noguera C. Interaction between oxygen vacancies on MgO. Phys Rev B, 1999, 59(7):5179[24] Gao B, Sun B, Zhang H, et al. Unified physical model of bipolar oxide based resistive switching memory. IEEE Electron Device Lett, 2009, 30:1326 doi: 10.1109/LED.2009.2032308[25] Wu X, Migas D B, Li X, et al. Role of oxygen vacancies in HfO2-based gate stack breakdown. Appl Phys Lett, 2010, 96:172901 doi: 10.1063/1.3416912 -
Proportional views





 DownLoad:
DownLoad: