| Citation: |
R. T. Sapkal, S. S. Shinde, K. Y. Rajpure, C. H. Bhosale. Photoelectrocatrocatalytic hydrolysis of starch by using sprayed ZnO thin films[J]. Journal of Semiconductors, 2013, 34(5): 053001. doi: 10.1088/1674-4926/34/5/053001
****
R. T. Sapkal, S. S. Shinde, K. Y. Rajpure, C. H. Bhosale. Photoelectrocatrocatalytic hydrolysis of starch by using sprayed ZnO thin films[J]. J. Semicond., 2013, 34(5): 053001. doi: 10.1088/1674-4926/34/5/053001.
|
Photoelectrocatrocatalytic hydrolysis of starch by using sprayed ZnO thin films
DOI: 10.1088/1674-4926/34/5/053001
More Information
-
Abstract
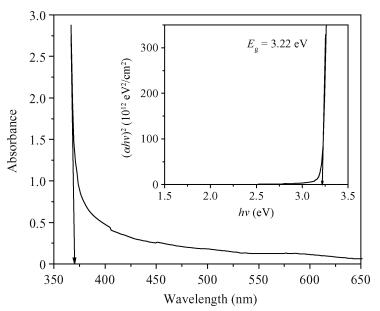
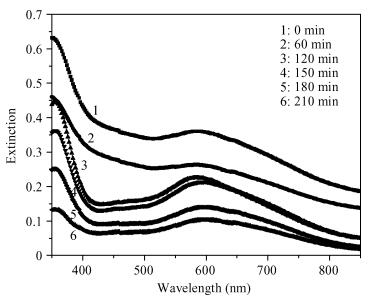
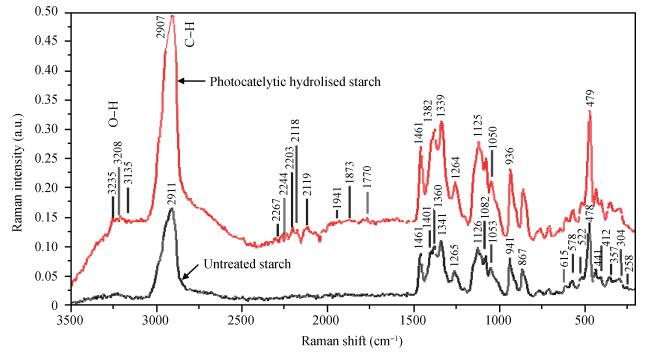
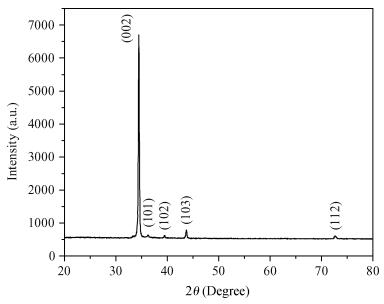
Thin films of zinc oxide have been deposited onto glass/FTO substrates at optimized 400℃ by using a chemical spray pyrolysis technique. Deposited films are characterized for their structural, morphological optical and photocatalytic activity by using XRD, an SEM, a UV-vis spectrophotometer, and a PEC single-cell reactor. Films are polycrystalline and have a hexagonal (wurtzite) crystal structure with c-axis (002) orientation growth perpendicular to the substrate surface. The observed direct band gap is about 3.22 eV for typical films prepared at 400℃. The photocatalytic activity of starch with a ZnO photocatalyst has been studied by using a novel photoelectrocatalytic process.-
Keywords:
- photoelectrocatalysis,
- hydrolysis,
- starch,
- DNSA
-
References
[1] Lee J B, Lee H J, Seo S H, et al. Characterization of undoped and Cu-doped ZnO films for surface acoustic wave applications. Thin Solid Films, 2001, 398/399:641[2] Lavrov R I, Ivon A I, Chernenko I M. Comparative characteristics of silver and copper electrodes on ZnO varistor ceramics. J Eur Cer Soc, 2004, 24:2591 doi: 10.1016/j.jeurceramsoc.2003.09.003[3] Minami T, Suzuki S, Miyata T. Transparent conducting impurity-co-doped ZnO:Al thin films prepared by magnetron sputtering. Thin Solid Films, 2001, 398/399:53[4] Caillaud F, Smith A, Baumard Chernenko J F. Deposition of ZnO films on polycrystalline alumina substrates by spray pyrolysis. J Eur Cer Soc, 1990, 6:313 doi: 10.1016/0955-2219(90)90022-8[5] Mende L S, Macmanus-Driscoll J L. ZnO——nanostructures, defects, and devices. Mater Today, 2007, 10:40[6] Gao W, Li Z. ZnO thin films produced by magnetron sputtering. Cera Inter, 2004, 30:1155 doi: 10.1016/j.ceramint.2003.12.197[7] Dai L, Deng H, Chen G, et al. Ultraviolet emission properties of ZnO film with zinc deficiency by SS CVD. Appl Surf Sci, 2008, 254:1599 doi: 10.1016/j.apsusc.2007.07.082[8] Mouet T, Devers T, Telia A, et al. Growth and characterization of thin ZnO films deposited on glass substrates by electrodeposition technique. Appl Surf Sci, 2010, 256:4114 doi: 10.1016/j.apsusc.2010.01.093[9] Shinde S S, Bhosale C H, Rajpure K Y. Photocatalytic degradation of toluene using sprayed N-doped ZnO thin films in aqueous suspension. J Photochem Photobiol B:Biol, 2012, 113:70 doi: 10.1016/j.jphotobiol.2012.05.008[10] Paraguay D F, Estrada L W, Acosta N D R, et al. Growth, structure and optical characterization of high quality ZnO thin films obtained by spray pyrolysis. Thin Solid Films, 1999, 350:192 doi: 10.1016/S0040-6090(99)00050-4[11] Bae H Y, Choi G M. Electrical and reducing gas sensing properties of ZnO and ZnO-CuO thin films fabricated by spin coating method. Sensors and Actuators B, 1999, 55:47 doi: 10.1016/S0925-4005(99)00038-6[12] Tamilarasan K, Ashok R, Abinandan S, et al. Optimization of operating variables for corn flour Starch hydrolysis using immobilized α-amylase by response surface methodology. Inter J Biotechnol Biochem, 2010, 6:841[13] Bej B, Basu R K, Ash S N. Kinetic studies on acid catalysed hydrolysis of starch. J Scientific and Industrial Res, 2008, 67:295[14] Shinde P S, Sadale S B, Patil P S, et al. UVA and solar light assisted photoelectrocatalytic degradation of AO7 dye in water using spray deposited TiO2 thin films. Appl Cat B:Environ, 2009, 89:288 doi: 10.1016/j.apcatb.2009.02.025[15] Fiedorowicz M, Lii C, Tomasik P. Physicochemical properties of potato starch illuminated with visible polarised light. Carbohydrate Polymers, 2002, 50:57 doi: 10.1016/S0144-8617(02)00012-7[16] Fiedorowicz M, Para A. Structural and molecular properties of dialdehyde starch. Carbohydrate Polymers, 2006, 63:360 doi: 10.1016/j.carbpol.2005.08.054[17] Ortega-Ojeda F E, Larsson H, Eliasson A C. Gel formation in mixtures of amylose and high amylopectin potato starch. Carbohydrate Polymers, 2004, 57:55 doi: 10.1016/j.carbpol.2004.03.024[18] Chiaramonte E, Rhazi L, Aussenac T, et al. Amylose and amylopectin in starch by asymmetric flow field-flow fractionation with multi-angle light scattering and refractive index detection (AF4-MALS-RI). J Cereal Sci, 2012, 56:457 doi: 10.1016/j.jcs.2012.04.006[19] Lide D R. Handbook of chemistry and physics. 87 Edn Boca Raton, FL:CRC Press, 1998 -
Proportional views





 DownLoad:
DownLoad: