| Citation: |
Yanwei Fan, Bukang Zhou, Junhua Wang, Zhaoyang Chen, Aimin Chang. Preparation and thermal-sensitive characteristic of copper doped n-type silicon material[J]. Journal of Semiconductors, 2015, 36(1): 013004. doi: 10.1088/1674-4926/36/1/013004
****
Y W Fan, B K Zhou, J H Wang, Z Y Chen, A M Chang. Preparation and thermal-sensitive characteristic of copper doped n-type silicon material[J]. J. Semicond., 2015, 36(1): 013004. doi: 10.1088/1674-4926/36/1/013004.
|
Preparation and thermal-sensitive characteristic of copper doped n-type silicon material
DOI: 10.1088/1674-4926/36/1/013004
More Information
-
Abstract
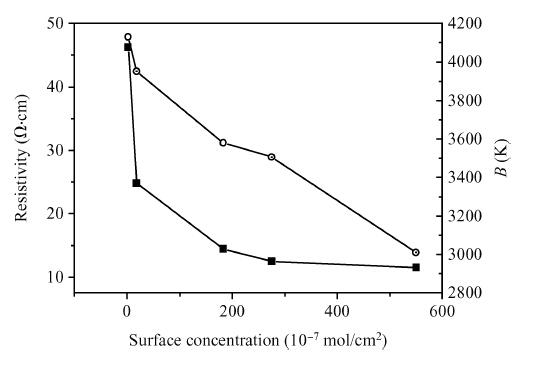
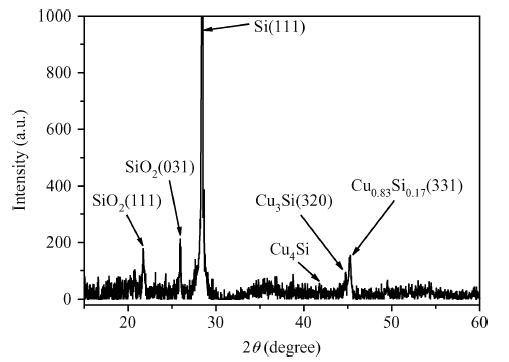
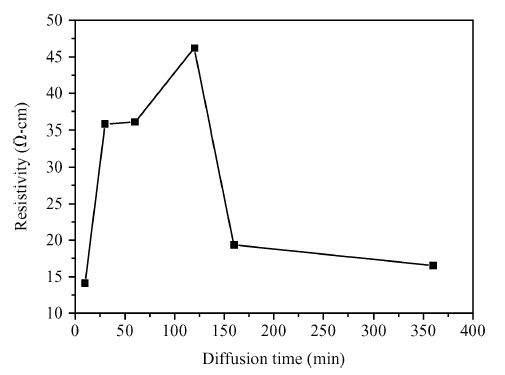
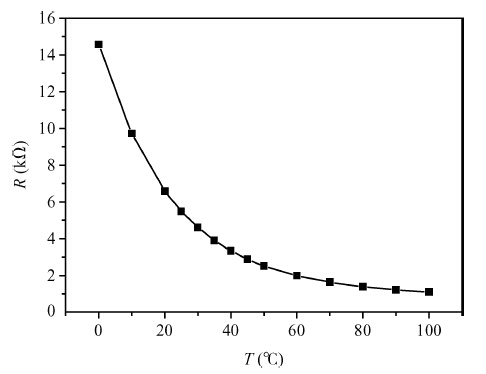
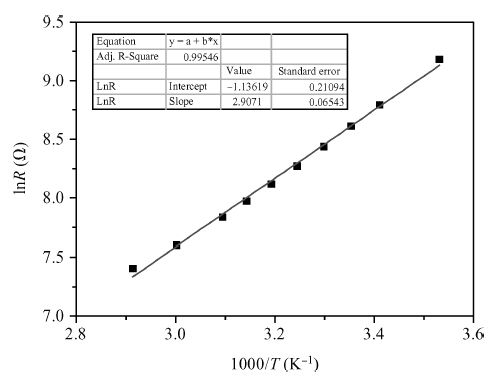
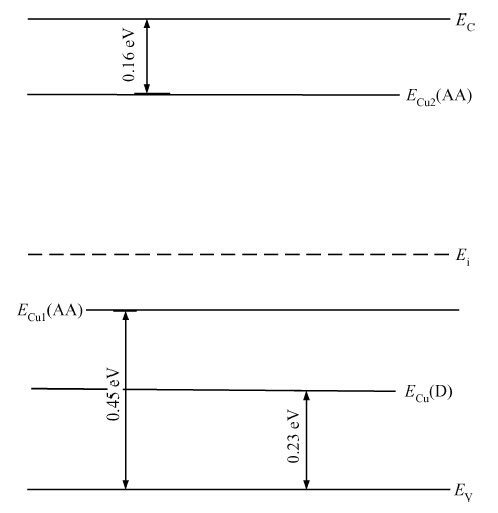
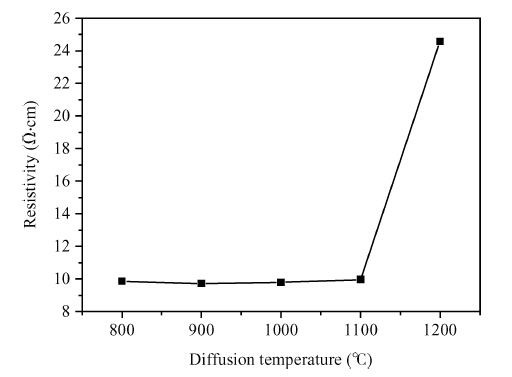
Copper doped n-type single-crystal silicon materials are prepared by a high temperature diffusion process. The electrical and thermal-sensitive characteristic of materials is investigated under different experimental conditions. The results show that the maximum resistivity of 46.2 Ω ·cm is obtained when the sample is treated at 1200 ℃ for 2 h with the surface concentration of the copper dopant source being 1.83 × 10-7 mol/cm2. The copper doped n-type silicon material presents a negative temperature-sensitive characteristic and the B values are about 3010-4130 K.-
Keywords:
- single-crystal silicon,
- deep level impurity,
- copper
-
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] -
Proportional views





 DownLoad:
DownLoad: