| Citation: |
K. Mahmood, N. Amin, A. Ali, M. Ajaz un Nabi, M. Imran Arshad, M. Zafar, M. Asghar. Enhancement of phosphors-solubility in ZnO by thermal annealing[J]. Journal of Semiconductors, 2015, 36(12): 123001. doi: 10.1088/1674-4926/36/12/123001
****
K. Mahmood, N. Amin, A. Ali, M. Ajaz un Nabi, M. Imran Arshad, M. Zafar, M. Asghar. Enhancement of phosphors-solubility in ZnO by thermal annealing[J]. J. Semicond., 2015, 36(12): 123001. doi: 10.1088/1674-4926/36/12/123001.
|
Enhancement of phosphors-solubility in ZnO by thermal annealing
DOI: 10.1088/1674-4926/36/12/123001
More Information
-
Abstract
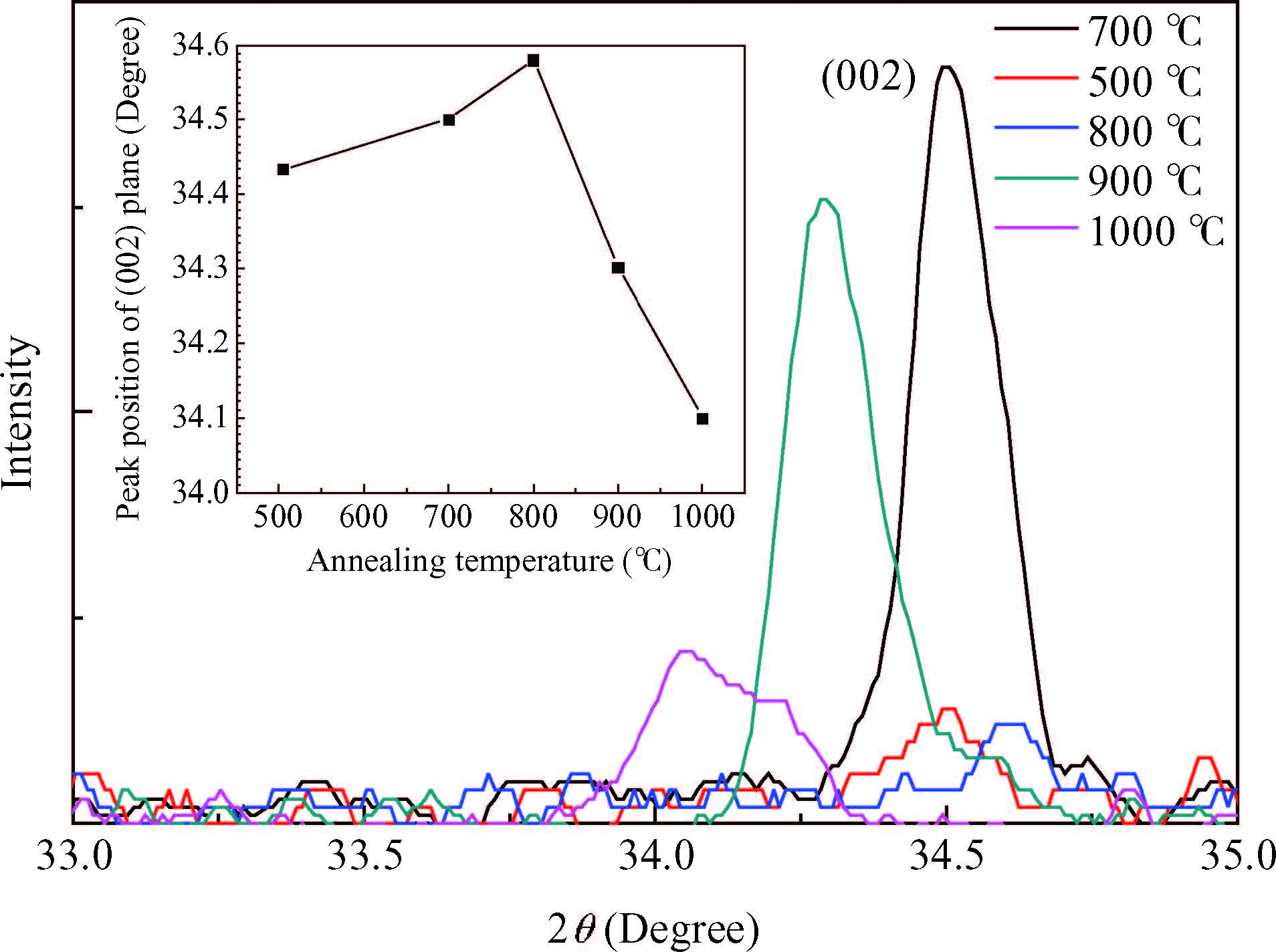
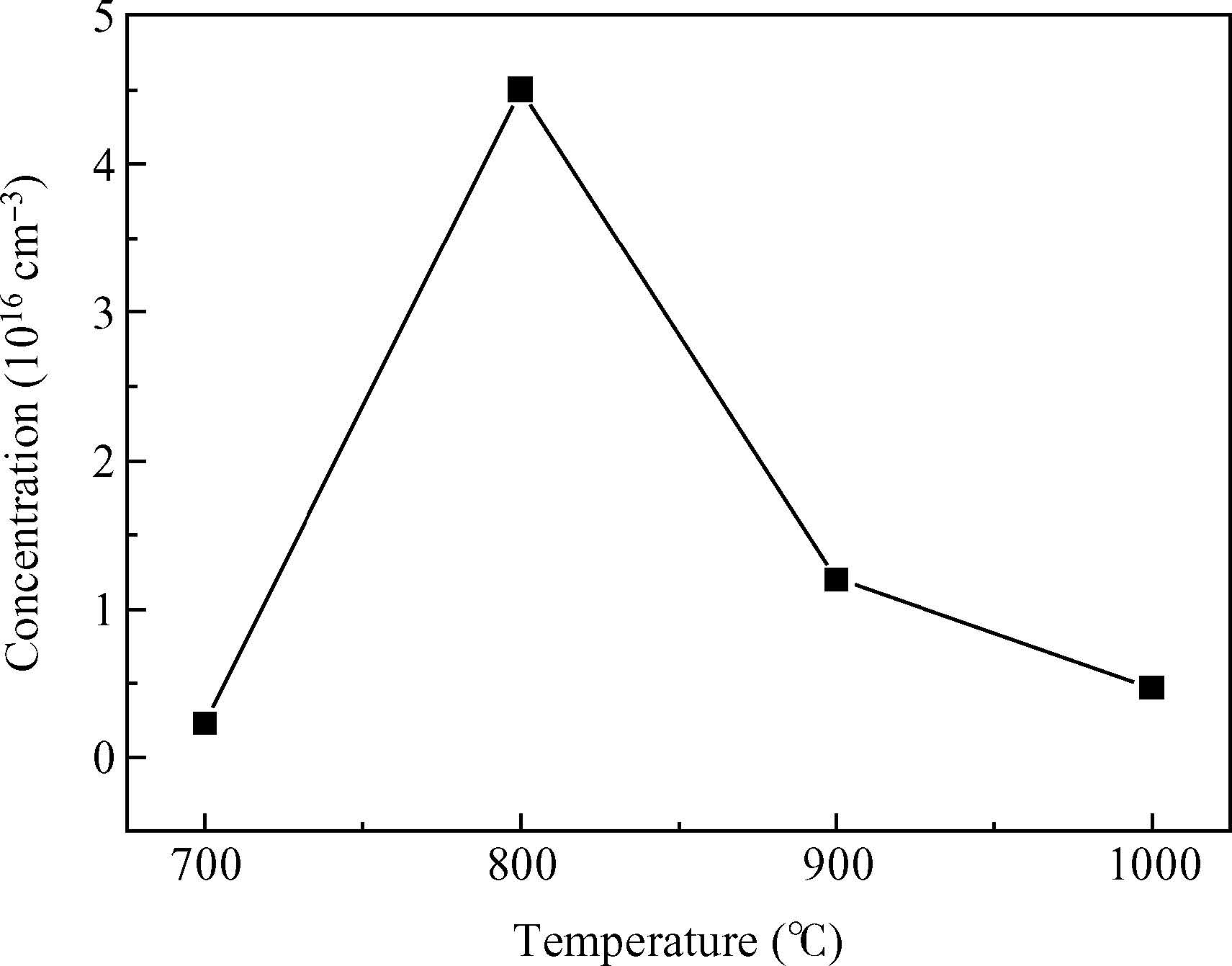
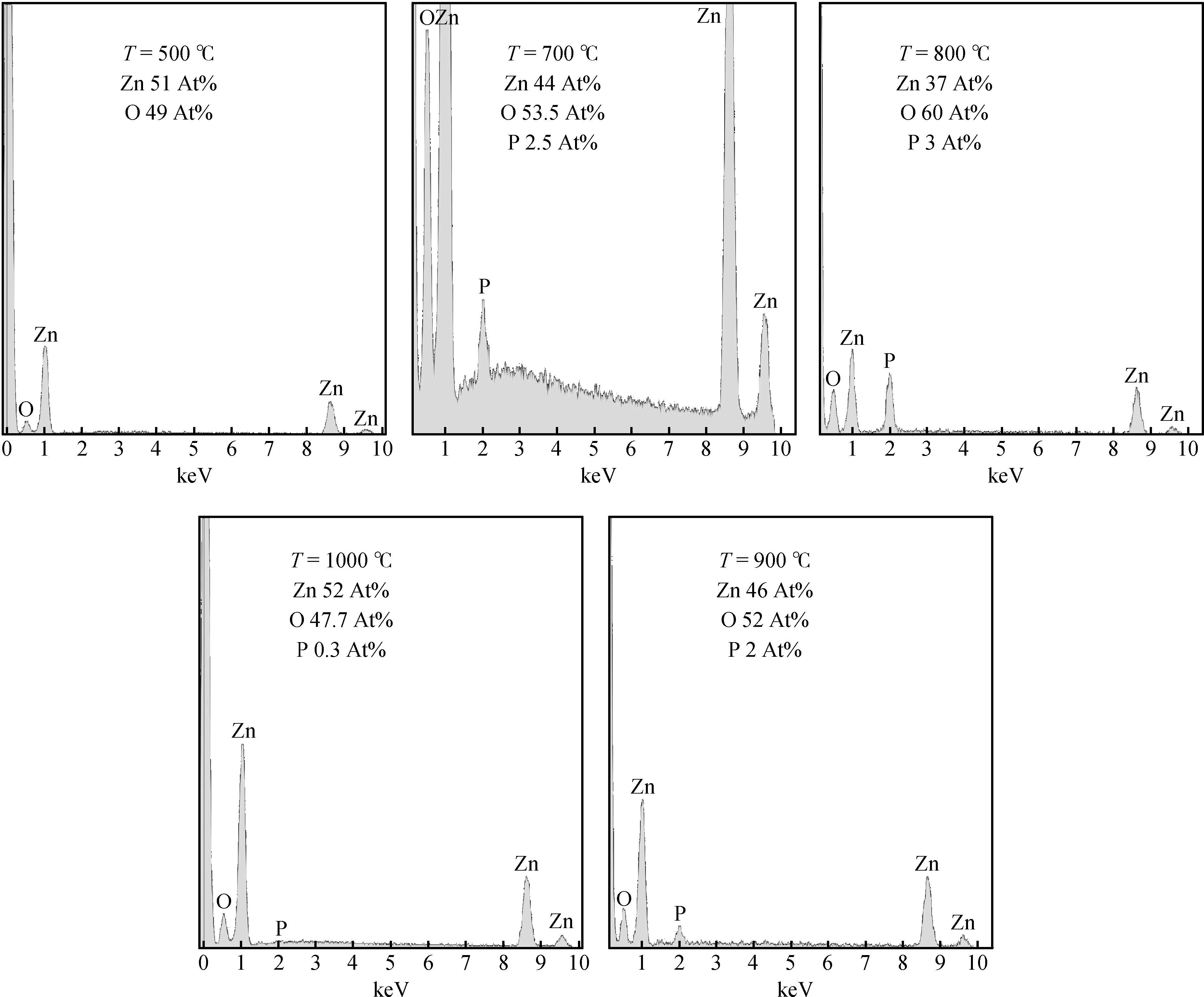
We have demonstrated the effect of annealing temperature on the diffusion density of phosphors in zinc oxide. The P-dopant P430 was sprayed on ZnO pellets and annealed at different temperatures from 500 to 1000℃ with a step of 100℃ for one hour using a programmable furnace. The concentration of P was controlled by varying the annealing temperature and the maximum solubility of P(3% At) was achieved at annealing 800℃ determined by energy dispersive X-ray diffraction(EDX) measurements. X-ray diffraction(XRD) confirmed the hexagonal structure of ZnO and showed that the growth direction was along the c-axis. We observed a maximum up shift in the(002) plane at an annealing temperature of 800℃, suggesting that P atoms replaced Zn atoms in the structure which results in the reduction of the lattice constant. Room temperature photoluminescence(PL) spectrum consists of a peak at 3.28 eV and related to band edge emission, but samples annealed at 800 and 900℃ have an additional donor acceptor pair peak at 3.2 eV. Hall effect measurements confirmed the p-type conductivity of the sample annealed at 800℃. -
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] -
Proportional views





 DownLoad:
DownLoad: