| Citation: |
Shuai Feng, Lichuan Zhao, Qingzhu Zhang, Pengpeng Yang, Zhaoyun Tang, Jiang Yan, Cinan Wu. Effects of a carbon implant on thermal stability of Ni0.95(Pt0.05)Si[J]. Journal of Semiconductors, 2015, 36(6): 063004. doi: 10.1088/1674-4926/36/6/063004
****
S Feng, L C Zhao, Q Z Zhang, P P Yang, Z Y Tang, J Yan, C N Wu. Effects of a carbon implant on thermal stability of Ni0.95(Pt0.05)Si[J]. J. Semicond., 2015, 36(6): 063004. doi: 10.1088/1674-4926/36/6/063004.
|
Effects of a carbon implant on thermal stability of Ni0.95(Pt0.05)Si
DOI: 10.1088/1674-4926/36/6/063004
More Information
-
Abstract
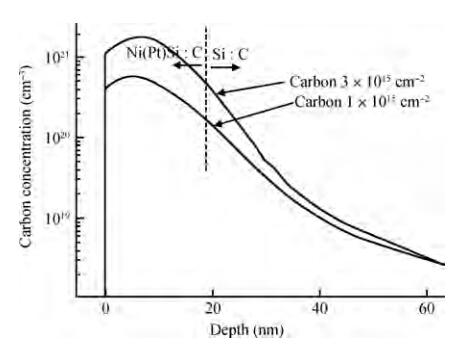
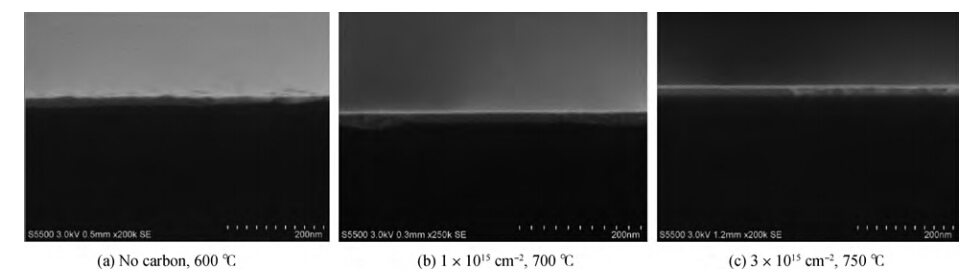
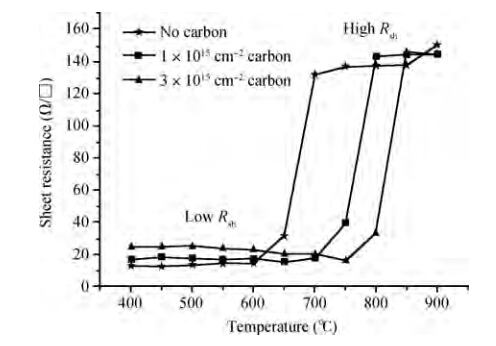
The effects of a carbon implant on thermal stability of Ni0.95(Pt0.05)Si films are investigated by implanting carbon of different doses into Si substrates before silicidation with two steps of rapid thermal annealing. Compared with the Ni0.95(Pt0.05)Si films without carbon implanting, the thermal stability of Ni0.95(Pt0.05)Si films with two carbon implant doses are improved 100 ℃ (1 × 1015cm-2) and 150 ℃ (3 × 1015cm-2-
Keywords:
- silicide,
- thermal stability,
- carbon implant,
- RTA
-
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] -
Proportional views





 DownLoad:
DownLoad: