| Citation: |
Haonan Zhang, Ming Zhuo, Yazi Luo, Yuejiao Chen. Mesoporous tin oxide nanospheres for a NOx in air sensor[J]. Journal of Semiconductors, 2017, 38(2): 023003. doi: 10.1088/1674-4926/38/2/023003
****
H N Zhang, M Zhuo, Y Z Luo, Y J Chen. Mesoporous tin oxide nanospheres for a NOx in air sensor[J]. J. Semicond., 2017, 38(2): 023003. doi: 10.1088/1674-4926/38/2/023003.
|
Mesoporous tin oxide nanospheres for a NOx in air sensor
DOI: 10.1088/1674-4926/38/2/023003
More Information
-
Abstract
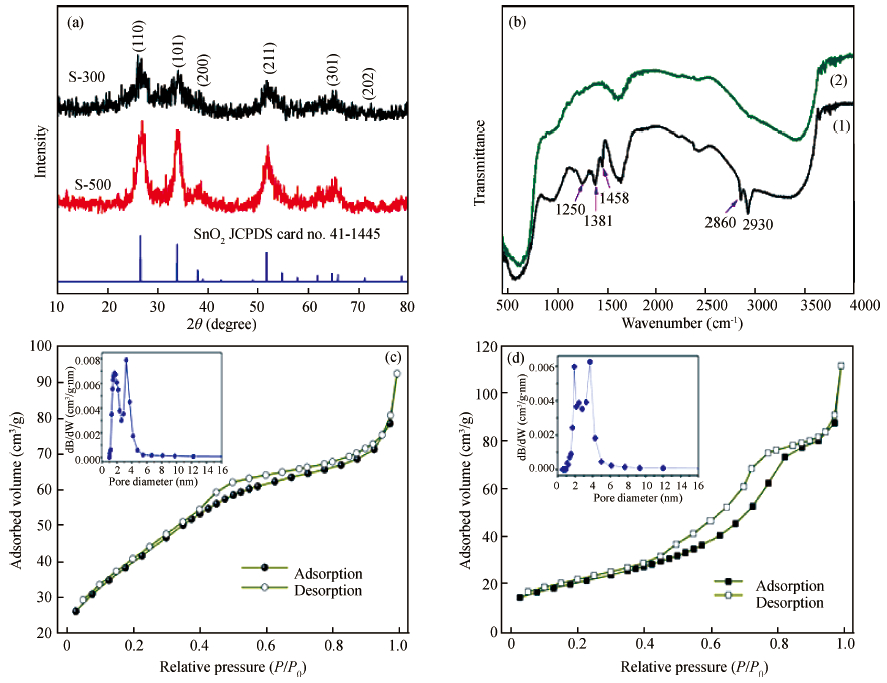
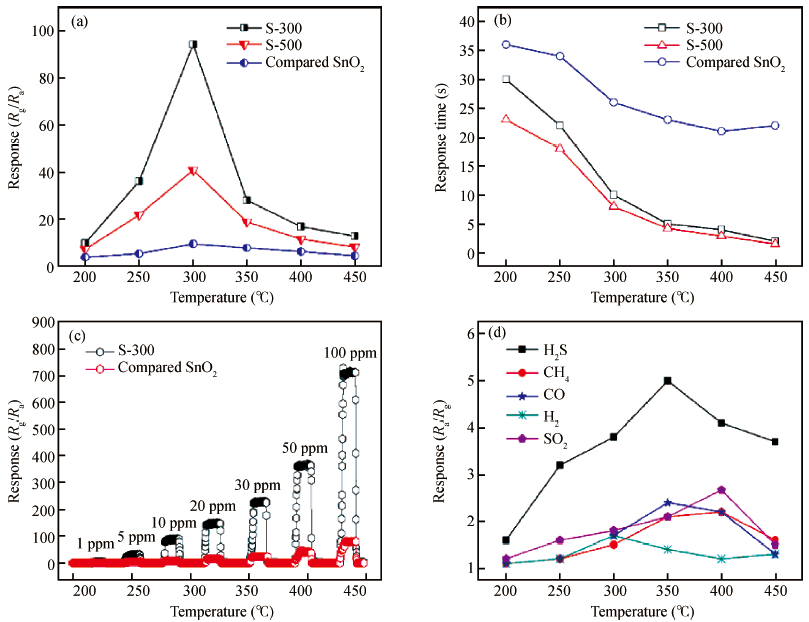
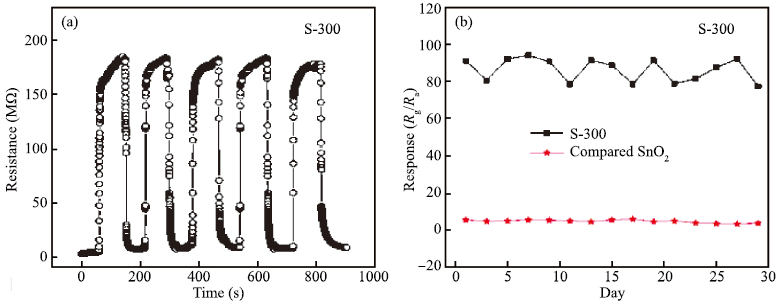
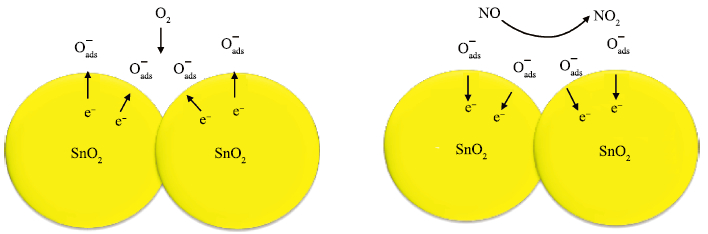
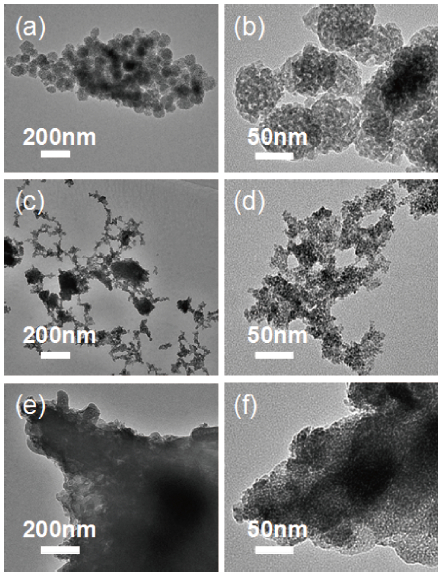
Mesoporous tin oxide(SnO2/with a high surface area of 147.5 m2/g has been successfully synthesized via self-assembly process, combining the driven forces of water-evaporation and molecular interactions. Scanning electron microscope, X-ray diffraction, transmission electron micrograph, Fourier transform infrared and BrunauerEmmett-Teller were employed to analyze the morphology and crystal structure of the as-synthesized mesoporous materials. As a gas sensor, mesoporous SnO2 shows impressive performances towards NOx gas with high selectivity and stability as well as ultra high sensitivity about 94.3 to 10 ppm NOx gas at 300℃. The best response time of the sample S-500 is about 3.4s to 10 ppm NOx at 450℃.-
Keywords:
- mesoporous materials,
- tin oxide,
- sensor,
- nanospheres
-
References
[1] Mei L, Chen Y, Ma J. Gas sensing of SnO2 nanocrystals revisited:developing ultra-sensitive sensors for detecting the H2S leakage of biogas. Sci Rep, 2014, 4:6028[2] Fan Z, Yan J, Zhi L, et al. A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors. Adv Mater, 2010, 22(33):3723 doi: 10.1002/adma.201001029[3] Wang D W, Li F, Zhao J, et al. Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano, 2009, 3(7):1745 doi: 10.1021/nn900297m[4] Kh S K, Noshin F, Khaulah S, et al. Sensitivity enhancement of OD- and OD-CNT-based humidity sensors by high gravity thin film deposition technique. J Semicond, 2015, 36(3):034005 doi: 10.1088/1674-4926/36/3/034005[5] Kim H J, Yoon J W, Choi K I, et al. Ultraselective and sensitive detection of xylene and toluene for monitoring indoor air pollution using Cr-doped NiO hierarchical nanostructures. Nanoscale, 2013, 5(15):7066 doi: 10.1039/c3nr01281f[6] Deng J, Ma J, Mei L, et al. Porous α-Fe2O3 nanosphere-based H2S sensor with fast response, high selectivity and enhanced sensitivity. J Maters Chem A, 2013, 1(40):12400 doi: 10.1039/c3ta12253k[7] Chi X, Liu C, Zhang J, et al. Toluene-sensing properties of In2O3 nanotubes synthesized by electrospinning. J Semicond, 2014, 35(6):064005 doi: 10.1088/1674-4926/35/6/064005[8] Hong Y, Liang T, Ge B, et al. A novel algorithmic method for piezoresistance calculation. J Semicond, 2014, 35(5):054009 doi: 10.1088/1674-4926/35/5/054009[9] Zhou L, Qin M, Chen S Q, et al. Ceramic thermal wind sensor based on advanced direct chip attaching package. J Semicond, 2014, 35(07):074015 doi: 10.1088/1674-4926/35/7/074015[10] Yang T, Lu B. Highly porous structure strategy to improve the SnO2 electrode performance for lithium-ion batteries. Phys Chem Chem Phys, 2014, 16(9):4115 doi: 10.1039/c3cp54144d[11] Ma J, Zhang J, Wang S, et al. Superior gas-sensing and lithiumstorage performance SnO2 nanocrystals synthesized by hydrothermal method. Cryst Eng Comm, 2011, 13(20):6077 doi: 10.1039/c1ce05320e[12] Kida T, DoiT, Shimanoe K. Synthesis of monodispersed SnO2 nanocrystals and their remarkably high sensitivity to volatile organic compounds. Chem Mater, 2010, 22(8):2662 doi: 10.1021/cm100228d[13] Zhuang Z, Huang F, Lin Z, et al. Aggregation-induced fast crystal growth of SnO2 nanocrystals. J Am Chem Soc, 2012, 134(39):16228 doi: 10.1021/ja305305r[14] Liu J, Li Y, Huang X, et al. Direct growth of SnO2 nanorod array electrodes for lithium-ion batteries. J Mater Chem, 2009, 19(13):1859 doi: 10.1039/b817036c[15] Cheng B, Russell J M, Shi W, et al. Large-scale, solution-phase growth of single-crystalline SnO2 nanorods. J Amn Chem Soc, 2004, 126(19):5972 doi: 10.1021/ja0493244[16] Xue X Y, He B, Yuan S, et al. SnO2/WO3 core-shell nanorods and their high reversible capacity as lithium-ion battery anodes. Nanotechnology, 2011, 22(39):395702 doi: 10.1088/0957-4484/22/39/395702[17] Xue X, Chen Z, Ma C, et al. One-step synthesis and gas-sensing characteristics of uniformly loaded Pt@SnO2 nanorods. J Phys Chem C, 2010, 114(9):3968 doi: 10.1021/jp908343r[18] Wang Y, Jiang X, Xia Y. A solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. J Am Chem Soc, 2003, 125(52):16176 doi: 10.1021/ja037743f[19] Fang X, Yan J, Hu L, et al. Thin SnO2 nanowires with uniform diameter as excellent field emitters:a stability of more than 2400 minutes. Adv Funct Mater, 2012, 22(8):1613 doi: 10.1002/adfm.v22.8[20] Ye J, Zhang H, Yang R, et al. Morphology-controlled synthesis of SnO2 nanotubes by using 1D silica mesostructures as sacrificial templates and their applications in lithium-ion batteries. Small, 2010, 6(2):296 doi: 10.1002/smll.v6:2[21] Li Y, Zhao Y, Zhang Z, et al. SnO2 nanobelts and nanocrystals:synthesis, characterization and optical properties. J Cryst Growth, 2008, 310(18):4226 doi: 10.1016/j.jcrysgro.2008.06.046[22] Xing L L, Yuan S, Chen Z H, et al. Enhanced gas sensing performance of SnO2/α-MoO3 heterostructure nanobelts. Nanotechnology, 2011, 22(22):225502 doi: 10.1088/0957-4484/22/22/225502[23] Li K M, Li Y J, Lu M Y, et al. Direct conversion of single-layer SnO nanoplates to multi-layer SnO2 nanoplates with enhanced ethanol sensing properties. Adv Funct Mater, 2009, 19(15):2453 doi: 10.1002/adfm.v19:15[24] Li F, Chen Y, Ma J. Porous SnO2 nanoplates for highly sensitive NO detection. J Mater Chem A, 2014, 2(20):7175 doi: 10.1039/c4ta00247d[25] Ding S, Chen J S, Qi G, et al. Formation of SnO2 hollow nanospheres inside mesoporous silica nanoreactors. J Am Chem Soc, 2010, 133(1):21 http://www.chemeurope.com/en/publications/25970/formation-of-sno2-hollow-nanospheres-inside-mesoporous-silica-nanoreactors.html[26] Wang Z, Luan D, Boey F Y C, et al. Fast formation of SnO2 nanoboxes with enhanced lithium storage capability. J Am Chem Soc, 2011, 133(13):4738 doi: 10.1021/ja2004329[27] Chen Y, Ma J, Yu L, et al. Mesoporous SnO2 nanospheres formed via a water-evaporating process with superior electrochemical properties. Cryst Eng Comm, 2012, 14(19):6170 doi: 10.1039/c2ce25769f[28] Guo W, Duan X, Shen Y, et al. Ionothermal synthesis of mesoporous SnO2 nanomaterials and their gas sensitivity depending on the reducing ability of toxic gases. Phys Chem Chem Phys, 2013, 15(27):11221 doi: 10.1039/c3cp51663f[29] Ramasamy E, Lee J. Ordered mesoporous SnO2-based photoanodes for high-performance dye-sensitized solar cells. J Phys Chem C, 2010, 114(50):22032 doi: 10.1021/jp1074797[30] Zhu P, Reddy M, Wu Y, et al. Mesoporous SnO2 agglomerates with hierarchical structures as an efficient dual-functional material for dye-sensitized solar cells. Chem Commun, 2012, 48(88):10865 doi: 10.1039/c2cc36049g[31] Recham N, Dupont L, Courty M, et al. Ionothermal synthesis of tailor-made LiFePO4 powders for Li-ion battery applications. Chem Mater, 2009, 21(6):1096 doi: 10.1021/cm803259x[32] Matsunaga N, Sakai G, Shimanoe K, et al. Formulation of gas diffusion dynamics for thin film semiconductor gas sensor based on simple reaction-diffusion equation. Sens Actuators B, 2003, 96(1):226 https://www.researchgate.net/profile/Naoki_Matsunaga/publication/245083393_Formulation_of_gas_diffusion_dynamics_for_thin_film_semiconductor_gas_sensor_based_on_simple_reactiondiffusion_equation/links/5640a5e508ae24cd3e408d04.pdf?inViewer=0&pdfJsDownload=0&origin=publication_detail[33] Florent M, Xue C, Zhao D, et al. Formation mechanism of cubic mesoporous carbon monolith synthesized by evaporationinduced self-assembly. Chem Mater, 2012, 24(2):383 doi: 10.1021/cm2032493[34] Henzie J, Grüwald M, Widmer-Cooper A, et al. Self-assembly of uniform polyhedral silver nanocrystals into densest packings and exotic superlattices. Nat Mater, 2012, 11(2):131 http://escholarship.org/uc/item/3fx5r22k[35] Ku J, Aruguete D M, Alivisatos A P, et al. Self-assembly of magnetic nanoparticles in evaporating solution. J Am Chem Soc, 2010, 133(4):838 http://www.docin.com/p-1547122358.html[36] Ma J, Duan X, Lian J, et al. Sb2S3 with various nanostructures:controllable synthesis, formation mechanism, and electrochemical performance toward lithium storage. Chem A Eur J, 2010, 16(44):13210 doi: 10.1002/chem.201000962[37] Miszta K, De Graaf J, Bertoni G, et al. Hierarchical self-assembly of suspended branched colloidal nanocrystals into superlattice structures. Nat Mater, 2011, 10(11):872 doi: 10.1038/nmat3121[38] Penza M, Tagliente M A, Mirenghi L, et al. Tungsten trioxide (WO3/sputtered thin films for a NOx gas sensor. Sens Actuators B, 1998, 50(1):9 doi: 10.1016/S0925-4005(98)00149-X[39] Spencer M J S, Yarovsky I. ZnO nanostructures for gas sensing:interaction of NO2, NO, O, and N with the ZnO(1010) surface. J Phys Chem C, 2010, 114(24):10881 doi: 10.1021/jp1016938[40] Comini E. Metal oxide nano-crystals for gas sensing. Anal Chim Acta, 2006, 568(1/2):28 http://cn.bing.com/academic/profile?id=e1e233ad8c04d59896e13863d2aef0e8&encoded=0&v=paper_preview&mkt=zh-cn[41] Barsan N, Weimar U. Conduction model of metal oxide gas sensors. J Electroceram, 2001, 7(3):143 doi: 10.1023/A:1014405811371[42] Tiemann M. Porous metal oxides as gas sensors. Chem A Eur J, 2007, 13(30):8376 doi: 10.1002/(ISSN)1521-3765[43] Jeon J M, Shim Y S, Han S D, et al. Vertically ordered SnO2 nanobamboos for substantially improved detection of volatile reducing gases. J Mater Chem A, 2015, 3(35):17939 doi: 10.1039/C5TA03293H[44] Cui Z M, Mechai A, Guo L, et al. Palladium nanoparticles on the inner wall of tin oxide hollow nanospheres with enhanced hydrogen sensing properties. RSC Adv, 2013, 3(35):14979 doi: 10.1039/c3ra41941j[45] Wang L, Fei T, Deng J, et al. Synthesis of rattle-type SnO2 structures with porous shells. J Mater Chem, 2012, 22(35):18111 doi: 10.1039/c2jm32520a[46] Shimizu Y, Egashira M. Basic aspects and challenges of semiconductor gas sensors. MRS Bull, 1999, 24(06):18 doi: 10.1557/S0883769400052465[47] Lv T, Chen Y, Ma J, et al. Hydrothermally processed SnO2 nanocrystals for ultrasensitive NO sensors. RSC Advances, 2014, 4(43):22487 doi: 10.1039/c4ra03121k -
Proportional views





 DownLoad:
DownLoad: