| Citation: |
Aihua Jia, Miao Kan, Jinping Jia, Yixin Zhao. Photodeposited FeOOH vs electrodeposited Co-Pi to enhance nanoporous BiVO4 for photoelectrochemical water splitting[J]. Journal of Semiconductors, 2017, 38(5): 053004. doi: 10.1088/1674-4926/38/5/053004
****
A H Jia, M Kan, J P Jia, Y X Zhao. Photodeposited FeOOH vs electrodeposited Co-Pi to enhance nanoporous BiVO4 for photoelectrochemical water splitting[J]. J. Semicond., 2017, 38(5): 053004. doi: 10.1088/1674-4926/38/5/053004.
|
Photodeposited FeOOH vs electrodeposited Co-Pi to enhance nanoporous BiVO4 for photoelectrochemical water splitting
DOI: 10.1088/1674-4926/38/5/053004
More Information
-
Abstract
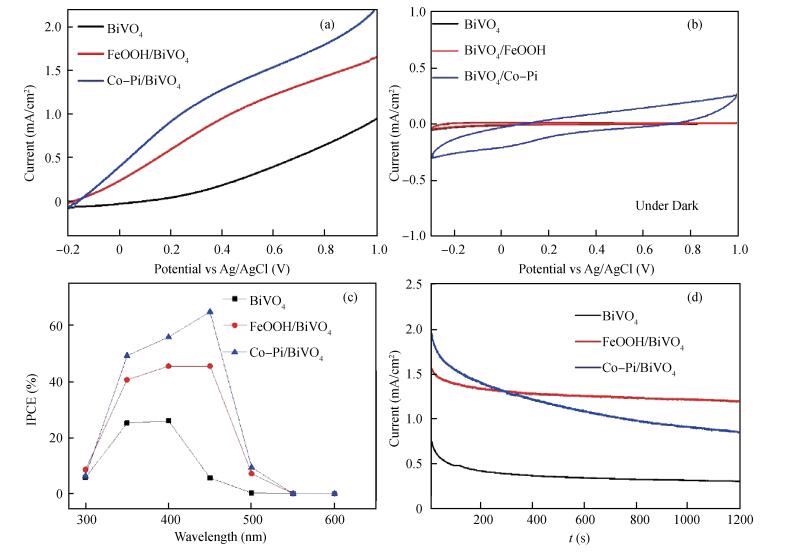
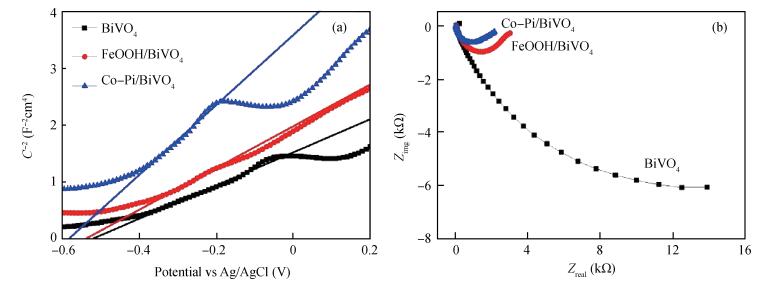
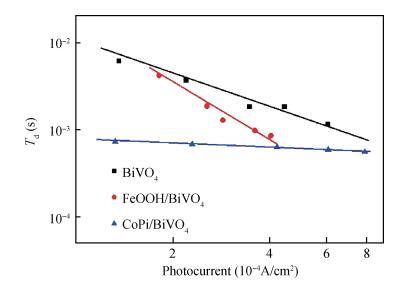
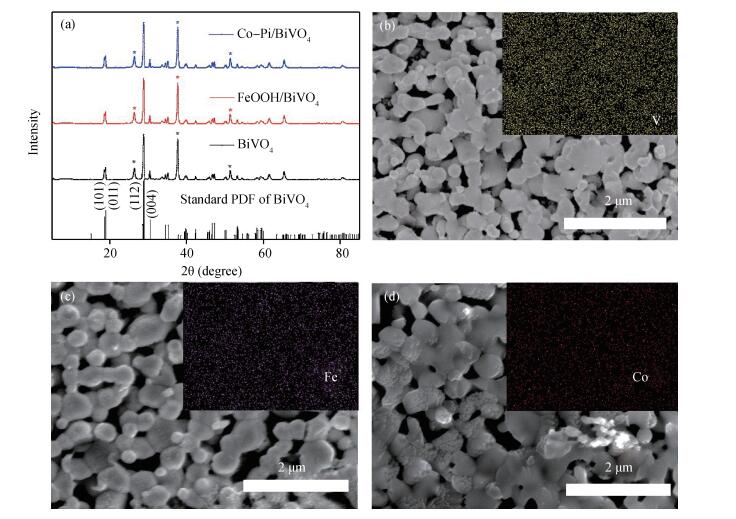
Co-Pi and FeOOH cocatalysts were in-situ deposited on the surface of nanoporous BiVO4 photoelectrodes. The FeOOH cocatalyst has little effect on the BiVO4 samples'morphologies, while the electrodeposited Co-Pi cocatalyst seems to affect the surface of BiVO4.The impedance intensity modulated photocurrent spectroscopy (IMPS), Mott-Schottky (M-S) techniques characterize BiVO4 samples photoelectrochemical performance with the deposition of Co-Pi and FeOOH.The Co-Pi/BiVO4 shows better photoelectrochemical performance than the FeOOH/BiVO4, but the FeOOH/BiVO4 exhibited the better stabilities.The flat band potential and slope of M-S plotof FeOOH/BiVO4 have little variations compared with BiVO4.In contrast, Co-Pi/BiVO4 exhibited the down shifted flat band potential, which is beneficial for the photoelectrochemical water oxidation.The electron transfer measurements revealed that the deposition of FeOOH and Co-Pi onto BiVO4 significantly enhanced the photoelectrochemical performance via reducing the interface resistance and promoting the electron transport.Furthermore, Co-Pi cocatalysts can further pin the transport-limiting traps and significantly facilitate the electron transport.-
Keywords:
- BiVO4,
- FeOOH,
- Co-Pi,
- water splitting
-
References
[1] Walter M G, Warren E L, Mckone J R, et al. Solar water splitting cells. Chem Rev, 2010, 110(11): 6646 https://core.ac.uk/display/4887222[2] Bak T, Nowotny J, Rekas M, et al. Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int J Hydrogen Energy, 2002, 27(10): 991 doi: 10.1016/S0360-3199(02)00022-8[3] Li J, Li L, Zheng L, et al. Photoelectrocatalytic degradation of rhodamine B using Ti/TiO2 electrode prepared by laser calcination method. Electrochim Acta, 2006, 51(23): 4942 doi: 10.1016/j.electacta.2006.01.037[4] Zhu N K K, Neale N R, Van De Lagemaat J, et al. Influence of surface area on charge transport and recombination in dye-sensitized TiO2 solar cells. J Phys Chem B, 2006, 110(50): 25174 doi: 10.1021/jp065284+[5] Halverson A F, Zhu K, Erslev P T, et al. Perturbation of the electron transport mechanism by proton intercalation in nanoporous TiO2 films. Nano Lett, 2012, 12(4): 2112 doi: 10.1021/nl300399w[6] Macak J M, Tsuchiya H, Ghicov A, et al. TiO2 nanotubes: self-organized electrochemical formation, properties and applications. Curr Opin Solid State Mater Sci, 2007, 11(1/2): 3 http://www.sciencedirect.com/science/article/pii/S1359028607000496[7] Hwang Y J, Hahn C, Liu B, et al. Photoelectrochemical properties of TiO2 nanowire arrays: a study of the dependence on length and atomic layer deposition coating. ACS Nano, 2012, 6(6): 5060 doi: 10.1021/nn300679d[8] Sang L, Zhao Y, Burda C. TiO2 nanoparticles as functional building blocks. Chem Rev, 2014, 114(19): 9283 doi: 10.1021/cr400629p[9] Ai G J, Li H X, Liu S P, et al. Solar water splitting by TiO2/CdS/Co-Pi nanowire array photoanode enhanced with Co-Pi as hole transfer relay and CdS as light absorber. Adv Funct Mater, 2015, 25(35): 5706 doi: 10.1002/adfm.201502461[10] Nguyen N T, Altomare M, Yoo J E, et al. Noble metals on anodic TiO2 nanotube mouths: thermal dewetting of minimal Pt Co-catalyst loading leads to significantly enhanced photocatalytic H2 generation. Adv Energy Mater, 2016, 6(2): 1501926 doi: 10.1002/aenm.201501926[11] Galembeck A, Alves O. BiVO4 thin film preparation by metal organic decomposition. Thin Solid Films, 2000, 365(1): 90 doi: 10.1016/S0040-6090(99)01079-2[12] Ke D, Peng T, Ma L, et al. Photocatalytic water splitting for O2 production under visible-light irradiation on BiVO4 nanoparticles in different sacrificial reagent solutions. Appl Catal A, 2008, 350(1): 111 doi: 10.1016/j.apcata.2008.08.003[13] Chemseddine A, Ullrich K, Mete T, et al. Solution-processed multilayered BiVO4 photoanodes: influence of intermediate heat treatments on the photoactivity. J Mater Chem A, 2016, 4(5): 1723 doi: 10.1039/C5TA08472E[14] Su J Z, Guo L J, Bao N Z, et al. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting. Nano Lett, 2011, 11(5): 1928 doi: 10.1021/nl2000743[15] Rossmeisl J, Qu Z W, Zhu H, et al. Electrolysis of water on oxide surfaces. J Electroanal Chem, 2007, 607(1/2): 83 https://www.researchgate.net/publication/229310161_Electrolysis_of_Water_on_Oxide_Surfaces[16] Lai Y H, Palm D W, Reisner E. Multifunctional coatings from scalable single source precursor chemistry in tandem photoelectrochemical water splitting. Adv Energy Mater, 2015, 5(24): 1501668 doi: 10.1002/aenm.201501668[17] Park Y, Mcdonald K J, Choi K S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem Soc Rev, 2013, 42(6): 2321 doi: 10.1039/C2CS35260E[18] Lettenmeier P, Wang L, Golla-Schindler U, et al. Nanosized IrO-Ir catalyst with relevant activity for anodes of proton exchange membrane electrolysis produced by a cost-effective procedure. Angew Chem Int Ed, 2015, 128(2): 752 https://www.researchgate.net/publication/284786466_Nanosized_IrO_x_-Ir_Catalyst_with_Relevant_Activity_for_Anodes_of_Proton_Exchange_Membrane_Electrolysis_Produced_by_a_Cost-Effective_Procedure[19] Zhao Y, Vargas-Barbosa N M, Strayer M E, et al. Understanding the effect of monomeric iridium (Ⅲ/Ⅳ) aquo complexes on the photoelectrochemistry of IrOx-nH2O-catalyzed water-splitting systems. J Am Chem Soc, 2015, 137(27): 8749 doi: 10.1021/jacs.5b03470[20] Lee Y, Suntivich J, May K J, et al. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions. J Phys Chem Lett, 2012, 3(3): 399 doi: 10.1021/jz2016507?src=recsys&journalCode=jpclcd[21] Ye H, Park H S, Bard A J. Screening of electrocatalysts for photoelectrochemical water oxidation on W-doped BiVO4 photocatalysts by scanning electrochemical microscopy. J Phys Chem C, 2011, 115(25): 12464 doi: 10.1021/jp200852c[22] Choi T W, Ka K S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science, 2014, 343(6174): 990 doi: 10.1126/science.1246913[23] Seabold J A, Zhu K, Neale N R. Efficient solar photoelectrolysis by nanoporous Mo:BiVO4 through controlled electron transport. Phys Chem Chem Phys, 2014, 16(3): 1121 doi: 10.1039/C3CP54356K[24] Pilli S K, Furtak T E, Brown L D, et al. Cobalt-phosphate (Co-Pi) catalyst modified Mo-doped BiVO4 photoelectrodes for solar water oxidation. Energy Environ Sci, 2011, 4(12): 5028 doi: 10.1039/c1ee02444b[25] Matthew W, Kanan D G N. In situ formation of an oxygen evolving catalyst in neutral water containing phosphate and Co2+. Science, 2008, 321(5892): 1072 doi: 10.1126/science.1162018[26] Abdi F F, Firet N, VanDeKrol R. Efficient BiVO4 thin film photoanodes modified with cobalt phosphate catalyst and W-doping. Chem Cat Chem, 2013, 5(2): 490 https://www.researchgate.net/publication/259295156_Efficient_BiVO4_Thin_Film_Photoanodes_Modified_with_Cobalt_Phosphate_Catalyst_and_W-doping[27] Kuang Y, Jia Q, Nishiyama H, et al. A front-illuminated nanostructured transparent BiVO4 photoanode for > 2% efficient water splitting. Adv Energy Mater, 2016, 6(2): 1501645 doi: 10.1002/aenm.201501645[28] Mcdonald K J, Choi K S. A new electrochemical synthesis route for a BiOI electrode and its conversion to a highly efficient porous BiVO4 photoanode for solar water oxidation. Energy Environ Sci, 2012, 5(9): 8553 doi: 10.1039/c2ee22608a[29] Zhong D K, Choi S, Gamelin D R. Near-complete suppression of surface recombination in solar photoelectrolysis by Co-Pi catalyst-modified W: BiVO4. J Am Chem Soc, 2011, 133(45): 18370 doi: 10.1021/ja207348x[30] Pilli S K, Deutsch T G, Furtak T E, et al. Light induced water oxidation on cobalt-phosphate (Co-Pi) catalyst modified semi-transparent, porous SiO2-BiVO4 electrodes. Phys Chem Chem Phys, 2012, 14(19): 7032 doi: 10.1039/c2cp40673j[31] Seabold J A, Neale N R. All first row transition metal oxide photoanode for water splitting based on Cu3V2O8. Chem Mater, 2015, 27(3): 1005 doi: 10.1021/cm504327f[32] Abdi F F, Van De Krol R. Nature and light dependence of bulk recombination in Co-Pi-catalyzed BiVO4. photoanodes. J Phys Chem C, 2012, 116(17): 9398 doi: 10.1021/jp3007552[33] Nair V, Perkins C L, Lin Q, et al. Textured nanoporous Mo:BiVO4 photoanodes with high charge transport and charge transfer quantum efficiencies for oxygen evolution. Energy Env-iron Sci, 2016, 9(4): 1412 doi: 10.1039/C6EE00129G[34] Berglund S P, Flaherty D W, Hahn N T, et al. Photoelectrochemical oxidation of water using nanostructured BiVO4 films. J Phys Chem C, 2011, 115(9): 3794 doi: 10.1021/jp1109459[35] Hong S J, Lee S, Jang J S, et al. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ Sci, 2011, 4(5): 1781 doi: 10.1039/c0ee00743a[36] Li W, He D, Sheehan S W, et al. Comparison of heterogenized molecular and heterogeneous oxide catalysts for photoelectrochemical water oxidation. Energy Environ Sci, 2016, 9(5): 1794 doi: 10.1039/C5EE03871E -
Proportional views





 DownLoad:
DownLoad: