| Citation: |
Jianqiang Qin, Lixiu Zhang, Chuantian Zuo, Zuo Xiao, Yongbo Yuan, Shangfeng Yang, Feng Hao, Ming Cheng, Kuan Sun, Qinye Bao, Zhengyang Bin, Zhiwen Jin, Liming Ding. A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency[J]. Journal of Semiconductors, 2021, 42(1): 010501. doi: 10.1088/1674-4926/42/1/010501
****
J Q Qin, L X Zhang, C T Zuo, Z Xiao, Y B Yuan, S F Yang, F Hao, M Cheng, K Sun, Q Y Bao, Z Y Bin, Z W Jin, L M Ding, A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency[J]. J. Semicond., 2021, 42(1): 010501. doi: 10.1088/1674-4926/42/1/010501.
|
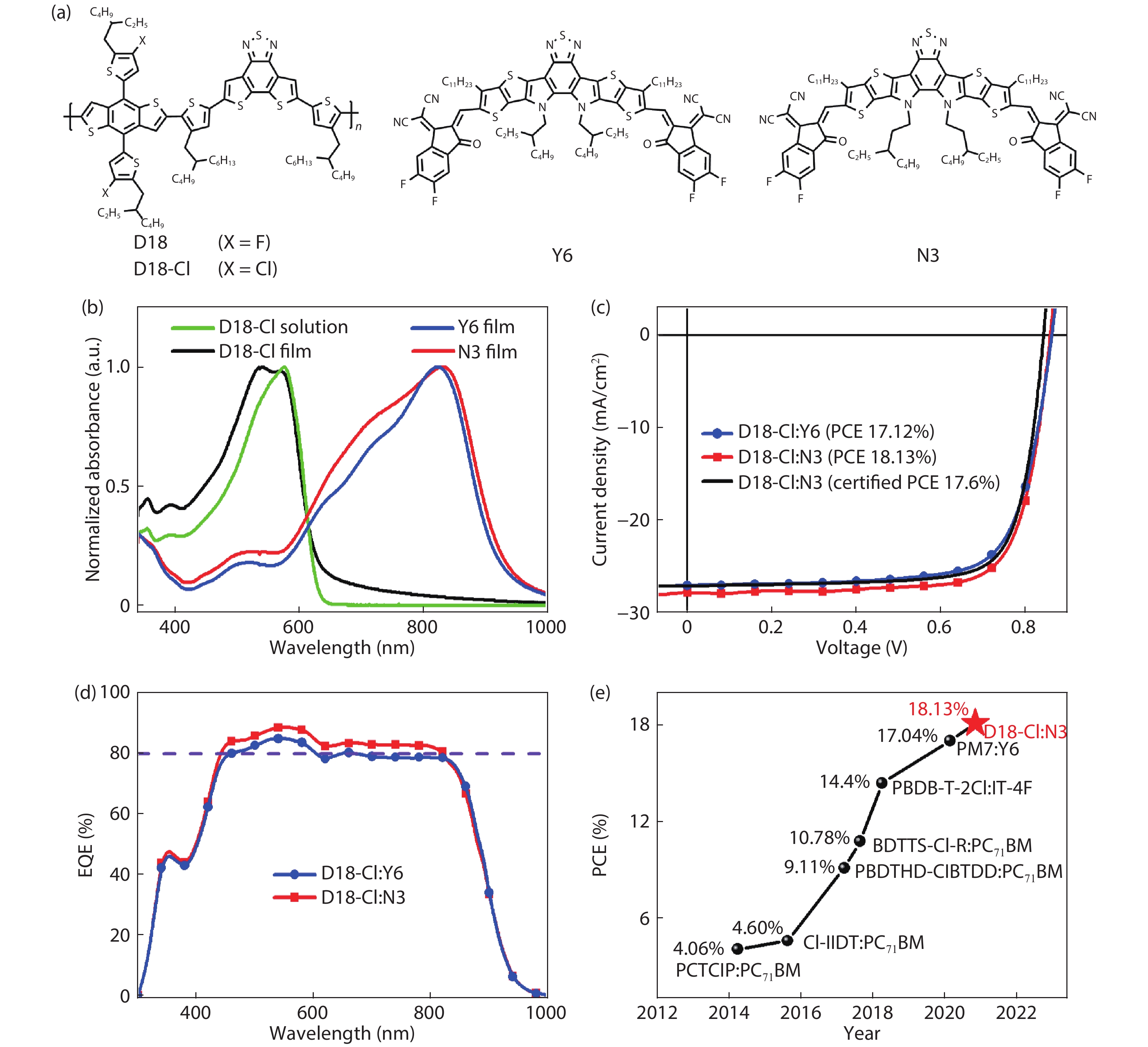
A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency
DOI: 10.1088/1674-4926/42/1/010501
More Information
-
References
[1] Lin Y, Wang J, Zhang Z, et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv Mater, 2015, 27, 1170 doi: 10.1002/adma.2014043217[2] Yan C, Barlow S, Wang Z, et al. Non-fullerene acceptors for organic solar cells. Nat Rev Mater, 2018, 3, 18003 doi: 10.1038/natrevmats.2018.3[3] Yuan J, Zhang Y, Zhou L, et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule, 2019, 3, 1140 doi: 10.1016/j.joule.2019.01.004[4] Liu Q, Jiang Y, Jin K, et al. 18% efficiency organic solar cells. Sci Bull, 2020, 65, 272 doi: 10.1016/j.scib.2020.01.001[5] Cui Y, Yao H, Zhang J, et al. Single-junction organic photovoltaic cells with approaching 18% efficiency. Adv Mater, 2020, 32, 1908205 doi: 10.1002/adma.201908205[6] Lin Y, Adilbekova B, Firdaus Y, et al. 17% efficient organic solar cells based on liquid exfoliated WS2 as a replacement for PEDOT: PSS. Adv Mater, 2019, 31, 1902965 doi: 10.1002/adma.201902965[7] Duan C, Ding L. The new era for organic solar cells: non-fullerene small molecular acceptors. Sci Bull, 2020, 65, 1231 doi: 10.1016/j.scib.2020.04.030[8] Duan C, Ding L. The new era for organic solar cells: polymer donors. Sci Bull, 2020, 65, 1422 doi: 10.1016/j.scib.2020.04.044[9] Xiao Z, Yang S, Yang Z, et al. Carbon-oxygen-bridged ladder-type building blocks for highly efficient nonfullerene acceptors. Adv Mater, 2019, 31, 1804790 doi: 10.1002/adma.201804790[10] Xiao Z, Liu F, Geng X, et al. A carbon-oxygen-bridged ladder-type building block for efficient donor and acceptor materials used in organic solar cells. Sci Bull, 2017, 62, 1331 doi: 10.1016/j.scib.2017.09.017[11] Xiao Z, Jia X, Li D, et al. 26 mA cm–2 Jsc from organic solar cells with a low-bandgap nonfullerene acceptor. Sci Bull, 2017, 62, 1494 doi: 10.1016/j.scib.2017.10.017[12] Xiao Z, Jia X, Ding L, et al. Ternary organic solar cells offer 14% power conversion efficiency. Sci Bull, 2017, 62, 1562 doi: 10.1016/j.scib.2017.11.003[13] Li H, Xiao Z, Ding L, et al. Thermostable single-junction organic solar cells with a power conversion efficiency of 14.62%. Sci Bull, 2018, 63, 340 doi: CNKI:SUN:JXTW.0.2018-06-004[14] Liu L, Liu Q, Xiao Z, et al. Induced J-aggregation in acceptor alloy enhances photocurrent. Sci Bull, 2019, 64, 1083 doi: CNKI:SUN:JXTW.0.2019-15-009[15] Wang T, Qin J, Xiao Z, et al. A 2.16 eV bandgap polymer donor gives 16% power conversion efficiency. Sci Bull, 2020, 65, 179 doi: 10.1016/j.scib.2019.11.030[16] Liu J, Liu L, Zuo C, et al. 5H-dithieno[3,2-b:2',3'-d]pyran-5-one unit yields efficient wide-bandgap polymer donors. Sci Bull, 2019, 64, 1655 doi: 10.1016/j.scib.2019.09.001[17] Xiong J, Jin K, Jiang Y, et al. Thiolactone copolymer donor gifts organic solar cells a 16.72% efficiency. Sci Bull, 2019, 64, 1573 doi: 10.1016/j.scib.2019.10.002[18] Wang T, Qin J, Xiao Z, et al. Multiple conformation locks gift polymer donor high efficiency. Nano Energy, 2020, 77, 105161 doi: 10.1016/j.nanoen.2020.105161[19] Liu Q, Jin K, Li W, et al. An efficient medium-bandgap nonfullerene acceptor for organic solar cells. J Mater Chem A, 2020, 8, 8857 doi: 10.1039/D0TA02427A[20] Xiong J, Xu J, Jiang Y, et al. Fused-ring bislactone building blocks for polymer donors. Sci Bull, 2020, 65, 1792 doi: 10.1016/j.scib.2020.07.018[21] Qin J, Zhang L, Xiao Z, et al. Over 16% efficiency from thick-film organic solar cells. Sci Bull, 2020, 65, 1979 doi: 10.1016/j.scib.2020.08.027[22] Brabec C J, Distler A, Du X, et al. Material strategies to accelerate OPV technology toward a GW technology. Adv Energy Mater, 2020, 10, 2001864 doi: 10.1002/aenm.202001864[23] Tong Y, Xiao Z, Du X, et al. Progress of the key materials for organic solar cells. Sci China Chem, 2020, 63, 758 doi: 10.1007/s11426-020-9726-0[24] Zhang M, Guo X, Ma W, et al. A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. Adv Mater, 2015, 27, 4655 doi: 10.1002/adma.201502110[25] Zhao W, Li S, Yao H, et al. Molecular optimization enables over 13% efficiency in organic solar cells. J Am Chem Soc, 2017, 139, 7148 doi: 10.1021/jacs.7b02677[26] Zhao Q, Qu J, He F. Chlorination: An effective strategy for high-performance organic solar cells. Adv Sci, 2020, 7, 2000509 doi: 10.1002/advs.202000509[27] Li Y, Meng B, Tong H, et al. A chlorinated phenazine-based donor-acceptor copolymer with enhanced photovoltaic performance. Polym Chem, 2014, 5, 1848 doi: 10.1039/C3PY01436C[28] Zheng Y Q, Wang Z, Dou J H, et al. Effect of halogenation in isoindigo-based polymers on the phase separation and molecular orientation of bulk heterojunction solar cells. Macromolecules, 2015, 48, 5570 doi: 10.1021/acs.macromol.5b01074[29] Mo D, Wang H, Chen H, et al. Chlorination of low-band-gap polymers: Toward high-performance polymer solar cells. Chem Mater, 2017, 29, 2819 doi: 10.1021/acs.chemmater.6b04828[30] Ji Z, Xu X, Zhang G, et al. Synergistic effect of halogenation on molecular energy level and photovoltaic performance modulations of highly efficient small molecular materials. Nano Energy, 2017, 40, 214 doi: 10.1016/j.nanoen.2017.08.027[31] Zhang S, Qin Y, Zhu J, et al. Over 14% efficiency in polymer solar cells enabled by a chlorinated polymer donor. Adv Mater, 2018, 30, 1800868 doi: 10.1002/adma.201800868[32] Ma R, Liu T, Luo Z, et al. Improving open-circuit voltage by a chlorinated polymer donor endows binary organic solar cells efficiencies over 17%. Sci China Chem, 2020, 63, 325 doi: 10.1007/s11426-019-9669-3[33] Chen H, Hu D, Yang Q, et al. All-small-molecule organic solar cells with an ordered liquid crystalline donor. Joule, 2019, 3, 3034 doi: 10.1016/j.joule.2019.09.009[34] Ye L, Xie Y, Weng K, et al. Insertion of chlorine atoms onto π-bridges of conjugated polymer enables improved photovoltaic performance. Nano Energy, 2019, 58, 220 doi: 10.1016/j.nanoen.2019.01.039[35] Su W, Li G, Fan Q, et al. Nonhalogen solvent-processed polymer solar cells based on chlorine and trialkylsilyl substituted conjugated polymers achieve 12.8% efficiency. J Mater Chem A, 2019, 7, 2351 doi: 10.1039/C8TA10662B[36] Tang A, Song W, Xiao B, et al. Benzotriazole-based acceptor and donors, coupled with chlorination, achieve a high VOC of 1.24 V and an efficiency of 10.5% in fullerene-free organic solar cells. Chem Mater, 2019, 31, 3941 doi: 10.1021/acs.chemmater.8b05316[37] Jeon S J, Han Y W, Moon D K. Chlorine effects of heterocyclic ring-based donor polymer for low-cost and high-performance nonfullerene polymer solar cells. Sol RRL, 2019, 3, 1900094 doi: 10.1002/solr.201970071[38] Wang T, Sun R, Xu S, et al. A wide-bandgap D-A copolymer donor based on a chlorine substituted acceptor unit for high performance polymer solar cells. J Mater Chem A, 2019, 7, 14070 doi: 10.1039/C9TA03272J[39] Huang J, Xie L, Hong L, et al. Significant influence of halogenation on the energy levels and molecular configurations of polymers in DTBDT-based polymer solar cells. Mater Chem Front, 2019, 3, 1244 doi: 10.1039/C9QM00212J[40] Wang Q, Li M, Zhang X, et al. Carboxylate-substituted polythiophenes for efficient fullerene-free polymer solar cells: The effect of chlorination on their properties. Macromolecules, 2019, 52, 4464 doi: 10.1021/acs.macromol.9b00793[41] Liao Z, Xie Y, Chen L, et al. Fluorobenzotriazole (FTAZ)-based polymer donor enables organic solar cells exceeding 12% efficiency. Adv Funct Mater, 2019, 29, 1808828 doi: 10.1002/adfm.201808828[42] Qin J, Lan L, Chen S, et al. Recent progress in flexible and stretchable organic solar cells. Adv Funct Mater, 2020, 30, 2002529 doi: 10.1002/adfm.202002529[43] Sun W, Zheng Y, Yang K, et al. Machine learning-assisted molecular design and efficiency prediction for high-performance organic photovoltaic materials. Sci Adv, 2019, 5, eaay4275 doi: 10.1126/sciadv.aay4275[44] Wang Z, Peng Z, Xiao Z, et al. Thermodynamic properties and molecular packing explain performance and processing procedures of three D18:NFA organic solar cells. Adv Mater, 2020, 32, 2005386 doi: 10.1002/adma.202005386[45] Jiang K, Wei Q, Lai J Y L, et al. Alkyl chain tuning of small molecule acceptors for efficient organic solar cells. Joule, 2019, 3, 3020 doi: 10.1016/j.joule.2019.09.010[46] Ziffer M E, Jo S B, Liu Y, et al. Tuning H- and J-aggregate behavior in π-conjugated polymers via noncovalent interactions. J Phys Chem C, 2018, 122, 18860 doi: 10.1021/acs.jpcc.8b05505[47] Xiao Z, Geng X, He D, et al. Development of isomer-free fullerene bisadducts for efficient polymer solar cells. Energy Environ Sci, 2016, 9, 2114 doi: 10.1039/C6EE01026A[48] An M, Xie F, Geng X, et al. A high-performance D-A copolymer based on dithieno[3,2-b:2',3'-d]pyridin-5(4H)-one unit compatible with fullerene and nonfullerene acceptors in solar cells. Adv Energy Mater, 2017, 7, 1602509 doi: 10.1002/aenm.201602509[49] Wang J, Gao Y, Xiao Z, et al. A wide-bandgap copolymer donor based on a phenanthridin-6(5H)-one unit. Mater Chem Front, 2019, 3, 2686 doi: 10.1039/C9QM00622B[50] Zhang L, Jin K, Xiao Z, et al. Alkoxythiophene and alkylthiothiophene π-bridges enhance the performance of A-D-A electron acceptors. Mater Chem Front, 2019, 3, 492 doi: 10.1039/C8QM00647D[51] Gao Y, Li D, Xiao Z, et al. High-performance wide-bandgap copolymers with dithieno[3,2-b:2',3'-d]pyridin-5(4H)-one units. Mater Chem Front, 2019, 3, 399 doi: 10.1039/C8QM00604K[52] Jin K, Deng C, Zhang L, et al. A heptacyclic carbon-oxygen-bridged ladder-type building block for A-D-A acceptors. Mater Chem Front, 2018, 2, 1716 doi: 10.1039/C8QM00285A[53] Li W, Liu Q, Jin K, et al. Fused-ring phenazine building blocks for efficient copolymer donors. Mater Chem Front, 2020, 4, 1454 doi: 10.1039/D0QM00080A -
Supplements
 20120021suppl.pdf
20120021suppl.pdf

-
Proportional views





 DownLoad:
DownLoad:
















