| Citation: |
Jiamin Cao, Guangan Nie, Lixiu Zhang, Liming Ding. Star polymer donors[J]. Journal of Semiconductors, 2022, 43(7): 070201. doi: 10.1088/1674-4926/43/7/070201
****
J M Cao, G A Nie, L X Zhang, L M Ding. Star polymer donors[J]. J. Semicond, 2022, 43(7): 070201. doi: 10.1088/1674-4926/43/7/070201
|
-
References
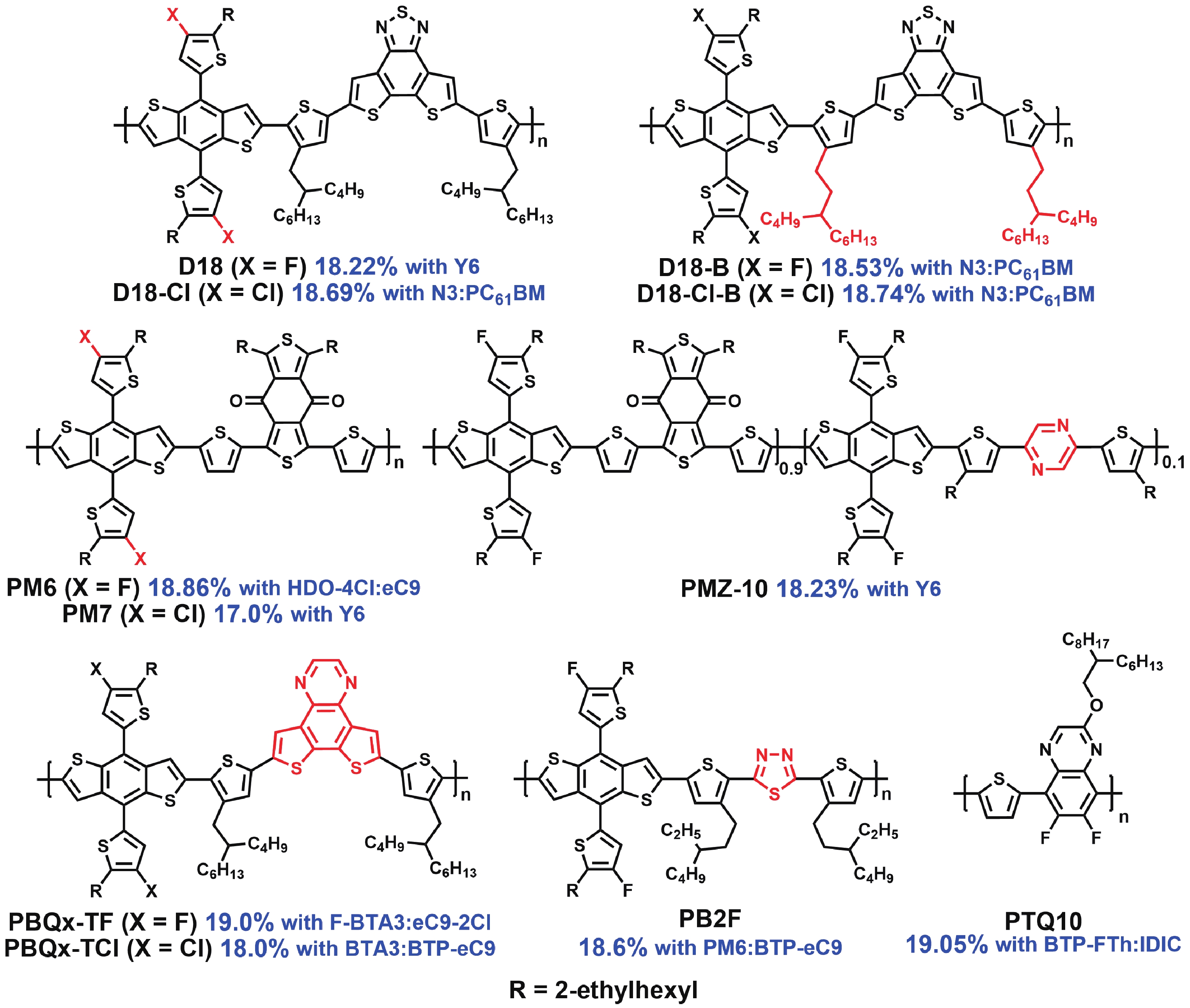
[1] Liu Q, Jiang Y, Jin K, et al. 18% Efficiency organic solar cells. Sci Bull, 2020, 65, 272 doi: 10.1016/j.scib.2020.01.001[2] Chong K, Xu X, Meng H, et al. Realizing 19.05% efficiency polymer solar cells by progressively improving charge extraction and suppressing charge recombination. Adv Mater, 2022, 34, 2109516 doi: 10.1002/adma.202109516[3] Zeng Y, Li D, Xiao Z, et al. Exploring the charge dynamics and energy loss in ternary organic solar cells with a fill factor exceeding 80%. Adv Energy Mater, 2021, 11, 2101338 doi: 10.1002/aenm.202101338[4] Li D, Zeng Y, Chen Z, et al. Investigating the reason for high FF from ternary organic solar cells. J Semicond, 2021, 42, 090501 doi: 10.1088/1674-4926/42/9/090501[5] Luo Y, Chen X, Xiao Z, et al. A large-bandgap copolymer donor for efficient ternary organic solar cells. Mater Chem Front, 2021, 5, 6139 doi: 10.1039/D1QM00835H[6] Duan C, Ding L. The new era for organic solar cells: non-fullerene small molecular acceptors. Sci Bull, 2020, 65, 1231 doi: 10.1016/j.scib.2020.04.030[7] Cao J, Yi L, Ding L. The origin and evolution of Y6 structure. J Semicond, 2022, 43, 030202 doi: 10.1088/1674-4926/43/3/030202[8] Wang J, Gao Y, Xiao Z, et al. A wide-bandgap copolymer donor based on a phenanthridin-6(5H)-one unit. Mater Chem Front, 2019, 3, 2686 doi: 10.1039/C9QM00622B[9] Wang T, Qin J, Xiao Z, et al. A 2.16 eV bandgap polymer donor gives 16% power conversion efficiency. Sci Bull, 2020, 65, 179 doi: 10.1016/j.scib.2019.11.030[10] Xiong J, Xu J, Jiang Y, et al. Fused-ring bislactone building blocks for polymer donors. Sci Bull, 2020, 65, 1792 doi: 10.1016/j.scib.2020.07.018[11] Jiang Y, Jin K, Chen X, et al. Post-sulphuration enhances the performance of a lactone polymer donor. J Semicond, 2021, 42, 070501 doi: 10.1088/1674-4926/42/7/070501[12] Ou Z, Qin J, Jin K, et al. Engineering of the alkyl chain branching point on a lactone polymer donor yields 17.81% efficiency. J Mater Chem A, 2022, 10, 3314 doi: 10.1039/D1TA10233H[13] Zheng Z, Yao H, Ye L, et al. PBDB-T and its derivatives: A family of polymer donors enables over 17% efficiency in organic photovoltaics. Mater Today, 2020, 35, 115 doi: 10.1016/j.mattod.2019.10.023[14] Ma R, Liu T, Luo Z, et al. Improving open-circuit voltage by a chlorinated polymer donor endows binary organic solar cells efficiencies over 17%. Sci China Chem, 2020, 63, 325 doi: 10.1007/s11426-019-9669-3[15] Zhou L, Meng L, Zhang J, et al. Introducing low-cost pyrazine unit into terpolymer enables high-performance polymer solar cells with efficiency of 18.23%. Adv Funct Mater, 2022, 32, 2109271 doi: 10.1002/adfm.202109271[16] Wang Z, Peng Z, Xiao Z, et al. Thermodynamic properties and molecular packing explain performance and processing procedures of three D18:NFA organic solar cells. Adv Mater, 2020, 32, 2005386 doi: 10.1002/adma.202005386[17] Qin J, Zhang L, Zuo C, et al. A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency. J Semicond, 2021, 42, 010501 doi: 10.1088/1674-4926/42/1/010501[18] Jin K, Xiao Z, Ding L. 18.69% PCE from organic solar cells. J Semicond, 2021, 42, 060502 doi: 10.1088/1674-4926/42/6/060502[19] Meng X, Jin K, Xiao Z, et al. Side chain engineering on D18 polymers yields 18.74% power conversion efficiency. J Semicond, 2021, 42, 100501 doi: 10.1088/1674-4926/42/10/100501[20] Li X, Xu J, Xiao Z, et al. Dithieno[3',2':3,4;2'',3'':5, 6]benzo[1,2-c][1,2,5]oxadiazole-based polymer donors with deep HOMO levels. J Semicond, 2021, 42, 060501 doi: 10.1088/1674-4926/42/6/060501[21] Sun A, Xu J, Zong G, et al. A wide-bandgap copolymer donor with a 5-methyl-4H-dithieno[3,2-e:2',3'-g]isoindole-4,6(5H)-dione unit. J Semicond, 2021, 42, 100502 doi: 10.1088/1674-4926/42/10/100502[22] Cui Y, Xu Y, Yao H, et al. Single-junction organic photovoltaic cell with 19% efficiency. Adv Mater, 2021, 33, 2102420 doi: 10.1002/adma.202102420[23] Xu Y, Cui Y, Yao H, et al. A new conjugated polymer that enables the integration of photovoltaic and light-emitting functions in one device. Adv Mater, 2021, 33, 2101090 doi: 10.1002/adma.202101090[24] Zhang T, An C, Bi P, et al. A thiadiazole-based conjugated polymer with ultradeep HOMO level and strong electroluminescence enables 18.6% efficiency in organic solar cell. Adv Energy Mater, 2021, 11, 2101705 doi: 10.1002/aenm.202101705[25] Duan C, Ding L. The new era for organic solar cells: polymer donors. Sci Bull, 2020, 65, 1422 doi: 10.1016/j.scib.2020.04.044[26] Qin J, Zhang L, Xiao Z, et al. Over 16% efficiency from thick-film organic solar cells. Sci Bull, 2020, 65, 1979 doi: 10.1016/j.scib.2020.08.027[27] Xu J, Sun A, Xiao Z, et al. Efficient wide-bandgap copolymer donor with reduced synthesis cost. J Mater Chem C, 2021, 9, 16187 doi: 10.1039/D1TC01746B[28] Yang X, Ding L. Organic semiconductors: commercialization and market. J Semicond, 2021, 42, 090201 doi: 10.1088/1674-4926/42/9/090201[29] Jin K, Ou Z, Zhang L, et al. A chlorinated lactone polymer donor featuring high performance and low cost. J Semicond, 2022, 43, 050501 doi: 10.1088/1674-4926/43/5/050501[30] Tong Y, Xiao Z, Du X, et al. Progress of the key materials for organic solar cells. Sci China Chem, 2020, 63, 758 doi: 10.1007/s11426-020-9726-0 -
Proportional views





 DownLoad:
DownLoad:














