| Citation: |
Yunye Wang, Zuo Xiao, Shanxin Xiong, Liming Ding. COF-based electrochromic materials and devices[J]. Journal of Semiconductors, 2022, 43(9): 090202. doi: 10.1088/1674-4926/43/9/090202
****
Y Y Wang, Z Xiao, S X Xiong, L M Ding. COF-based electrochromic materials and devices[J]. J. Semicond, 2022, 43(9): 090202. doi: 10.1088/1674-4926/43/9/090202
|
-
References
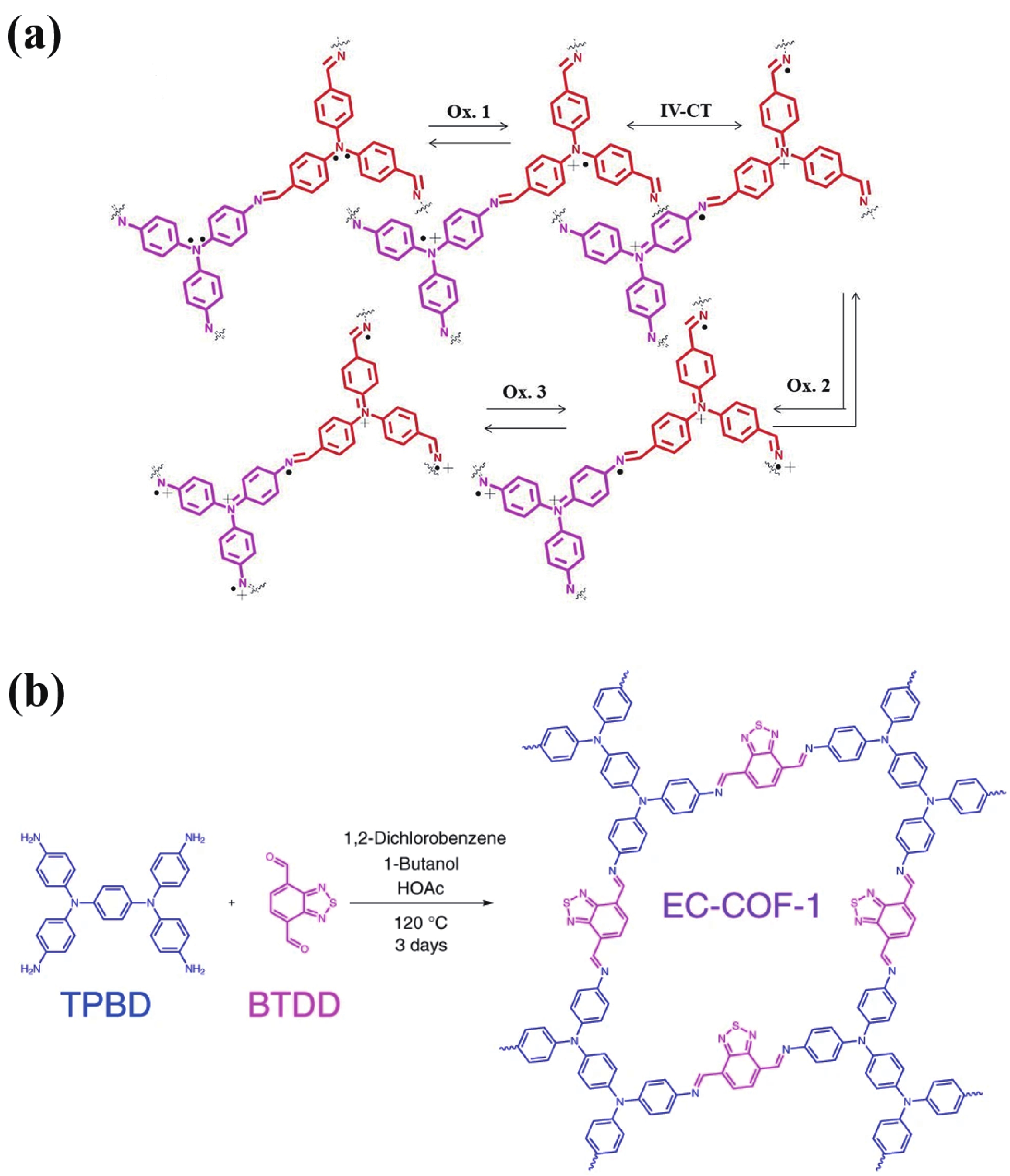
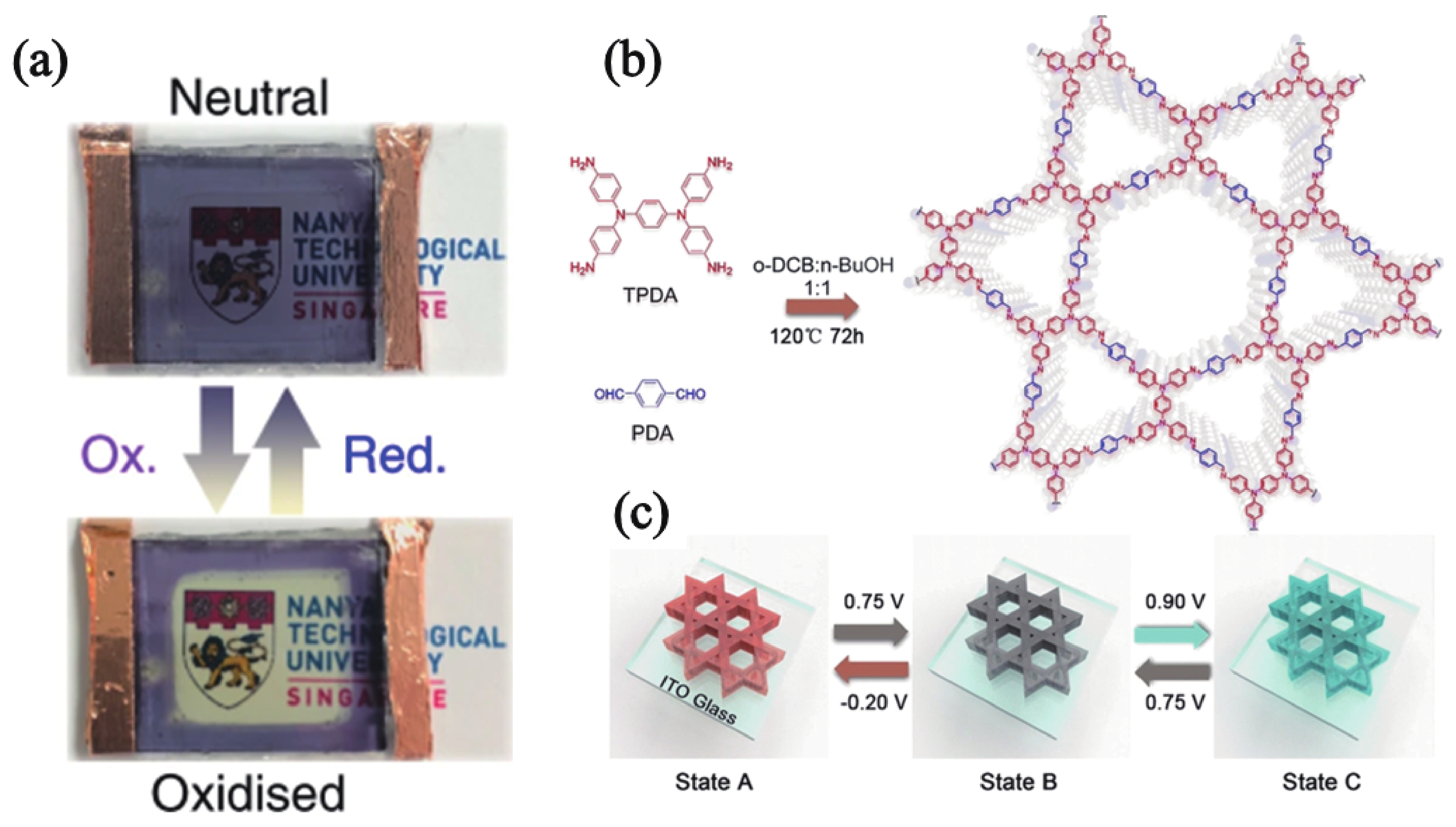
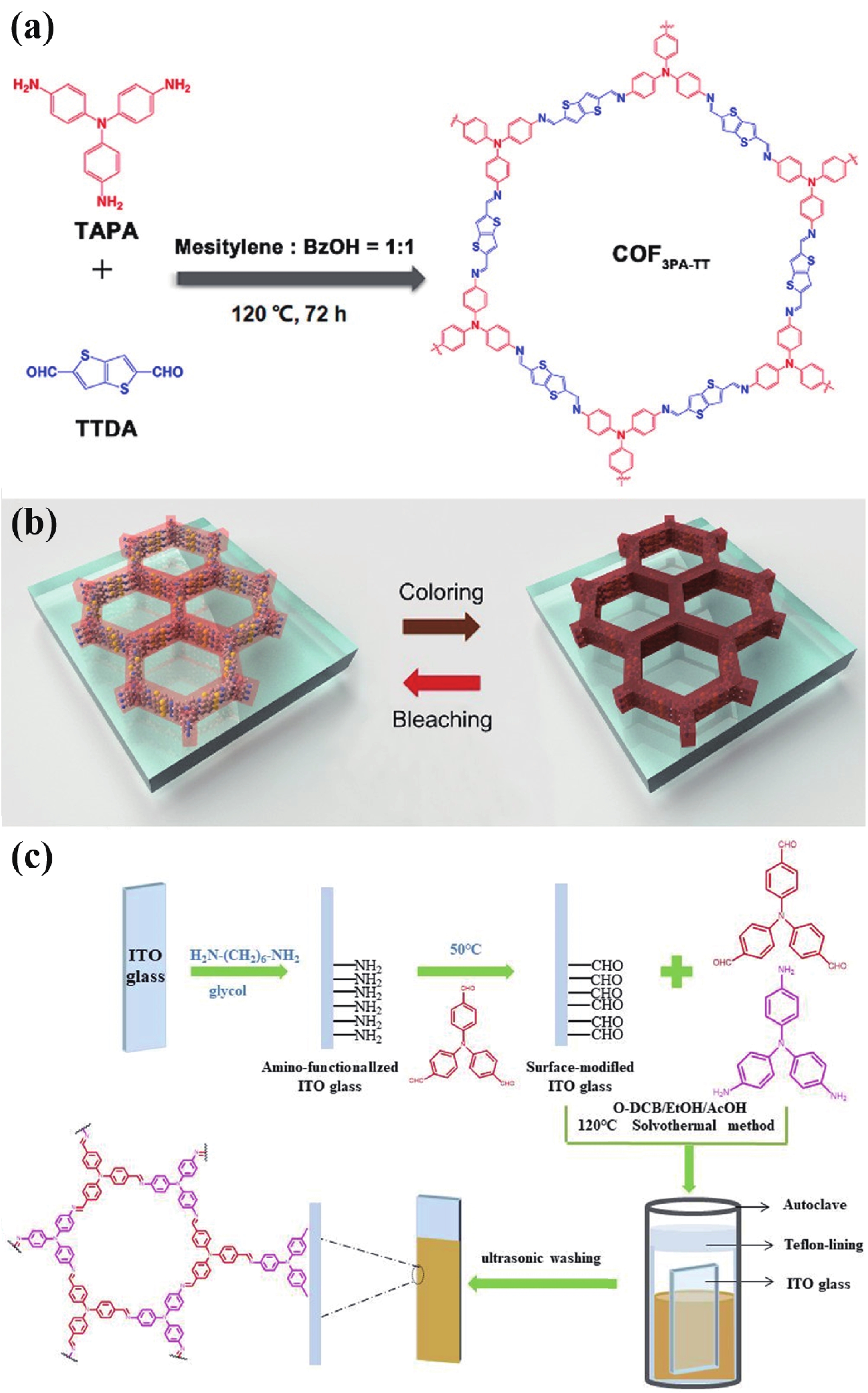
[1] Thakur V K, Ding G, Ma J, et al. Hybrid materials and polymer electrolytes for electrochromic device applications. Adv Mater, 2012, 24, 4071 doi: 10.1002/adma.201200213[2] Gu H, Ming S, Lin K, et al. Isoindigo as an electron-deficient unit for high-performance polymeric electrochromics. Electrochim Acta, 2018, 260, 772 doi: 10.1016/j.electacta.2017.12.033[3] Arockiam J B, Son H, Han S H, et al. Iron phthalocyanine incorporated metallo-supramolecular polymer for superior electrochromic performance with high coloration efficiency and switching stability. ACS Appl Energy Mater, 2019, 2, 8416 doi: 10.1021/acsaem.9b01022[4] Xie Y X, Zhao W N, Li G C, et al. A naphthalenediimide-based metal-organic framework and thin film exhibiting photochromic and electrochromic properties. Inorg Chem, 2016, 55, 549 doi: 10.1021/acs.inorgchem.5b02480[5] Furukawa S, Ashburne J. Greater porosity with redox reaction speeds up MOF color change. Chem, 2016, 1, 186 doi: 10.1016/j.chempr.2016.07.002[6] Geng K, He T, Liu R, et al. Covalent organic frameworks: design, synthesis, and functions. Chem Rev, 2020, 120, 8814 doi: 10.1021/acs.chemrev.9b00550[7] Cao S, Li B, Zhu R, et al. Design and synthesis of covalent organic frameworks towards energy and environment fields. Chem Eng J, 2019, 355, 602 doi: 10.1016/j.cej.2018.08.184[8] Chen X, Geng K, Liu R, et al. Covalent organic frameworks: chemical approaches to designer structures and built-in functions. Angew Chem Int Ed, 2020, 59, 5050 doi: 10.1002/anie.201904291[9] Bessinger D, Muggli K, Beetz M, et al. Fast-switching vis-IR electrochromic covalent organic frameworks. J Am Chem Soc, 2021, 143, 7351 doi: 10.1021/jacs.0c12392[10] Segura J L, Mancheño M J, Zamora F. Covalent organic frameworks based on Schiff-base chemistry: synthesis, properties and potential applications. Chem Soc Rev, 2016, 45, 5635 doi: 10.1039/C5CS00878F[11] Yang L, Guo Q, Kang H, et al. Self-controlled growth of covalent organic frameworks by repolymerization. Chem Mater, 2020, 32, 5634 doi: 10.1021/acs.chemmater.0c01140[12] Hao Q, Li Z J, Lu C, el al. Oriented two-dimensional covalent organic framework films for near-infrared electrochromic application. J Am Chem Soc, 2019, 141, 19831 doi: 10.1021/jacs.9b09956[13] Yen H J, Liou G S. Recent advances in triphenylamine-based electrochromic derivatives and polymers. Polym Chem, 2018, 9, 3001 doi: 10.1039/C8PY00367J[14] Xiong S, Wang Y, Wang X, et al. Schiff base type conjugated organic framework nanofibers: Solvothermal synthesis and electrochromic properties. Sol Energy Mater Sol Cells, 2020, 209, 110438 doi: 10.1016/j.solmat.2020.110438[15] Yu F, Liu W, Ke S W, et al. Electrochromic two-dimensional covalent organic framework with a reversible dark-to-transparent switch. Nat Commun, 2020, 11, 5534 doi: 10.1038/s41467-020-19315-6[16] Hao Q, Li Z J, Bai B, et al. A covalent organic framework film for three-state near-infrared electrochromism and a molecular logic gate. Angew Chem Int Ed, 2021, 133, 12606 doi: 10.1002/ange.202100870[17] Lv F, Xiong S, Zhang J, et al. Enhanced electrochromic properties of 2,6-diaminoanthraquinone and 1,3,5-triformylresorcinol (DAAQ-TFP) covalent organic framework/functionalized graphene oxide composites containing anthraquinone active unit. Electrochim Acta, 2021, 398, 139301 doi: 10.1016/j.electacta.2021.139301[18] Xiong S, Zhang Y, Zhang J, et al. Solvothermal synthesis and enhanced electrochromic properties of covalent organic framework/functionalized carbon nanotubes composites electrochromic materials with anthraquinonoid active unit. Sol Energy Mater Sol Cells, 2022, 235, 111489 doi: 10.1016/j.solmat.2021.111489 -
Proportional views





 DownLoad:
DownLoad: