| Citation: |
Tong Hoang Lin, Che Quang Cong, Nguyen Thanh Hoai Nam, Hoang An, Nguyen Duy Hai, Ton That Buu, Thoi Le Nhat Binh, Hoang Le Minh, Lam Thanh Ngan, Hoang Thuy Kim Ngan, Du Chi Vi, Ta Dang Khoa, Nguyen Huu Hieu. Green synthesis of three-dimensional magnesium ferrite/titanium dioxide/reduced graphene from Garcinia mangostana extract for crystal violet photodegradation and antibacterial activity[J]. Journal of Semiconductors, 2023, 44(12): 122702. doi: 10.1088/1674-4926/44/12/122702
****
T H Lin, C Q Cong, N T H Nam, H An, N D Hai, T T Buu, T L N Binh, H L Minh, L T Ngan, H T K Ngan, D C Vi, T D Khoa, N H Hieu. Green synthesis of three-dimensional magnesium ferrite/titanium dioxide/reduced graphene from Garcinia mangostana extract for crystal violet photodegradation and antibacterial activity[J]. J. Semicond, 2023, 44(12): 122702. doi: 10.1088/1674-4926/44/12/122702
|
Green synthesis of three-dimensional magnesium ferrite/titanium dioxide/reduced graphene from Garcinia mangostana extract for crystal violet photodegradation and antibacterial activity
DOI: 10.1088/1674-4926/44/12/122702
More Information
-
Abstract
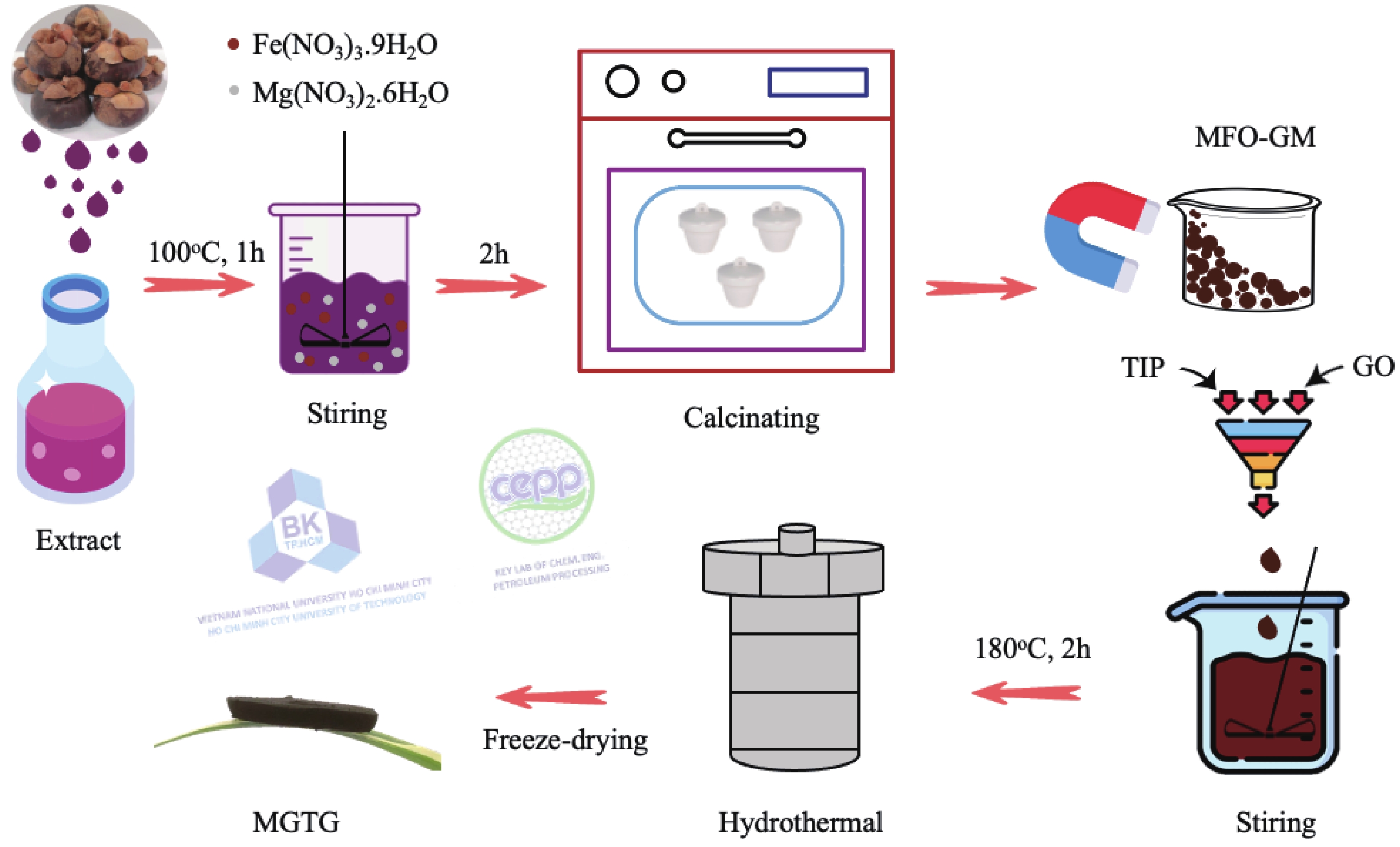
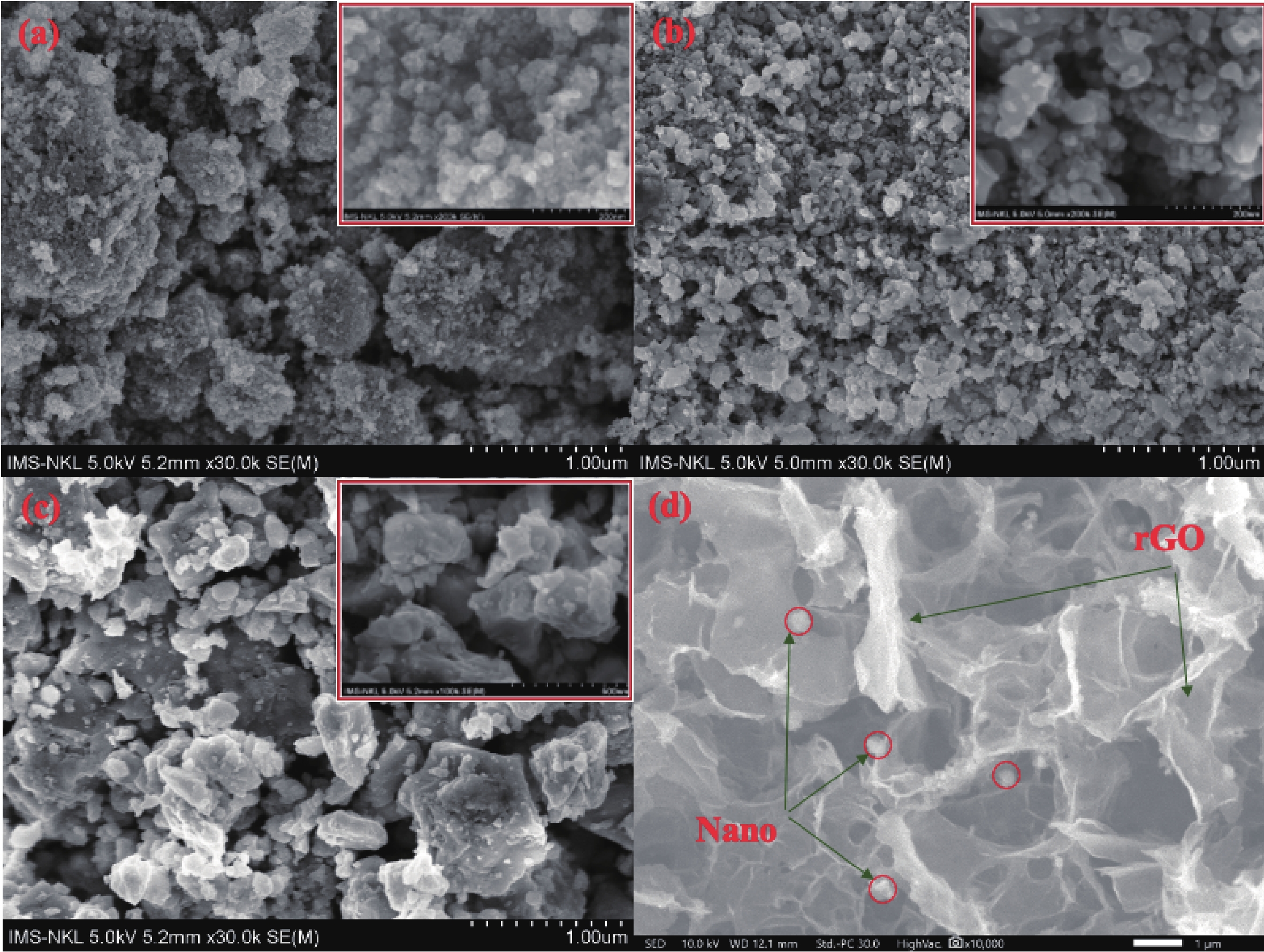
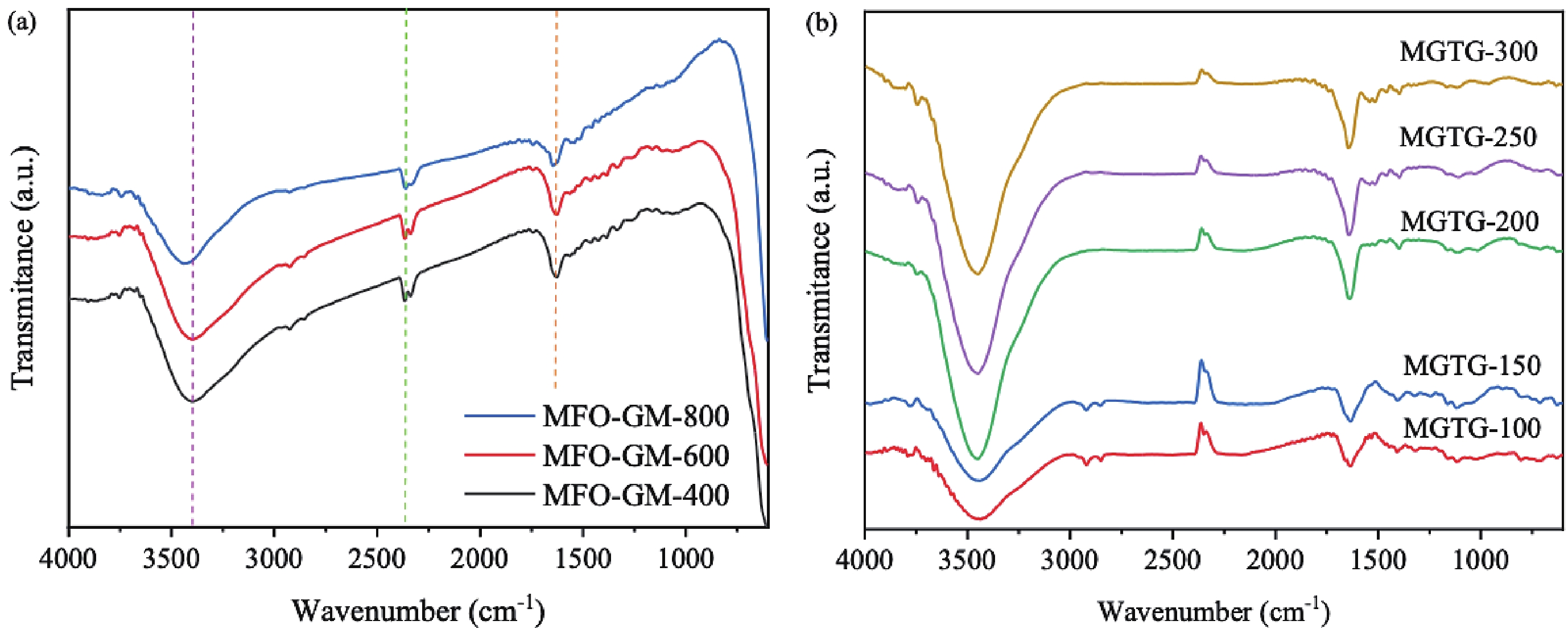
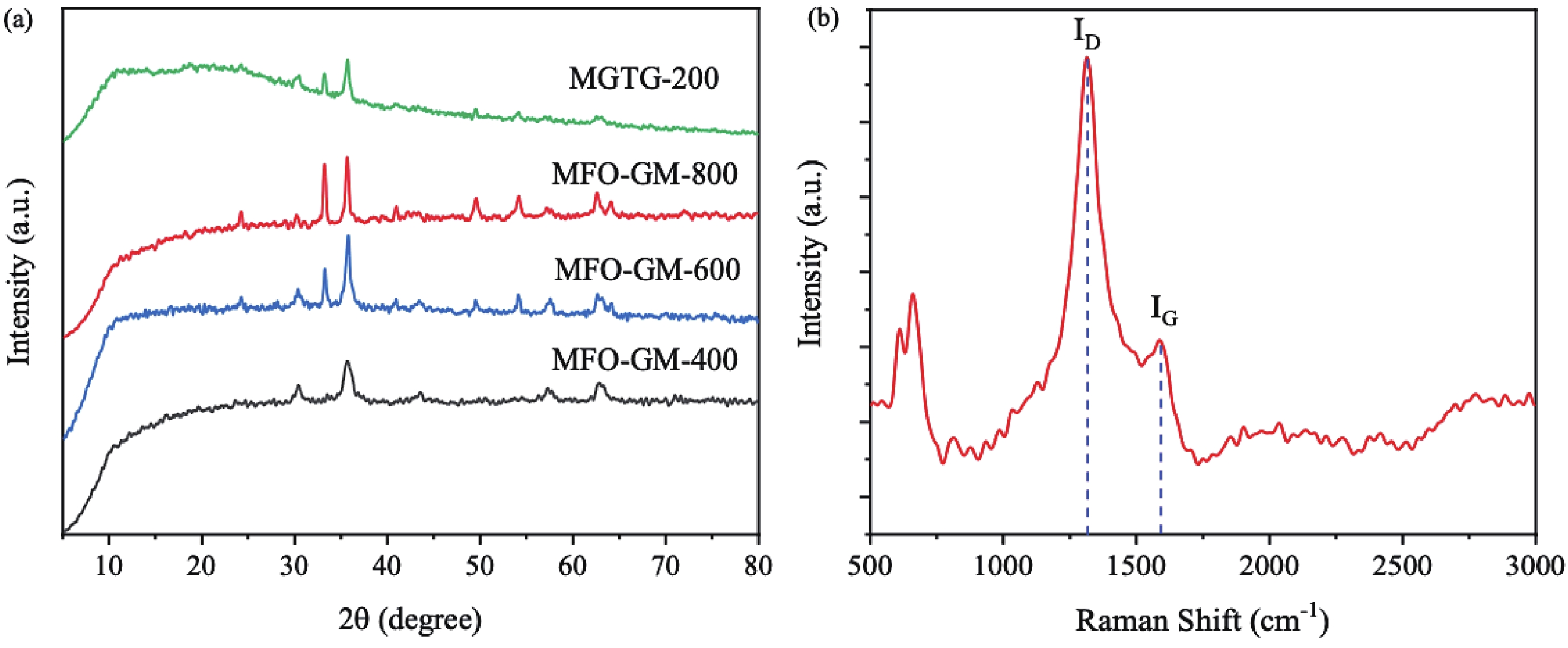
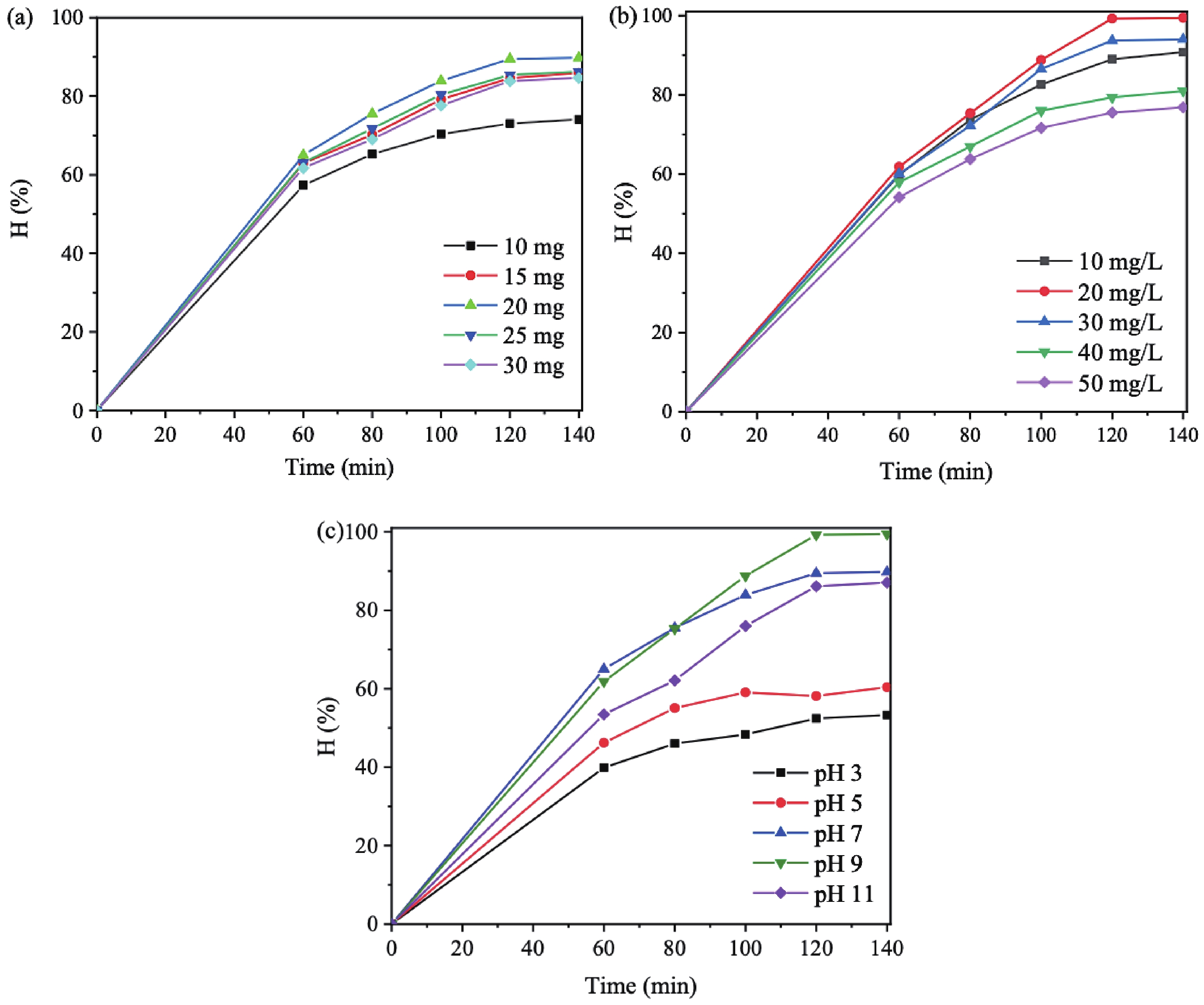
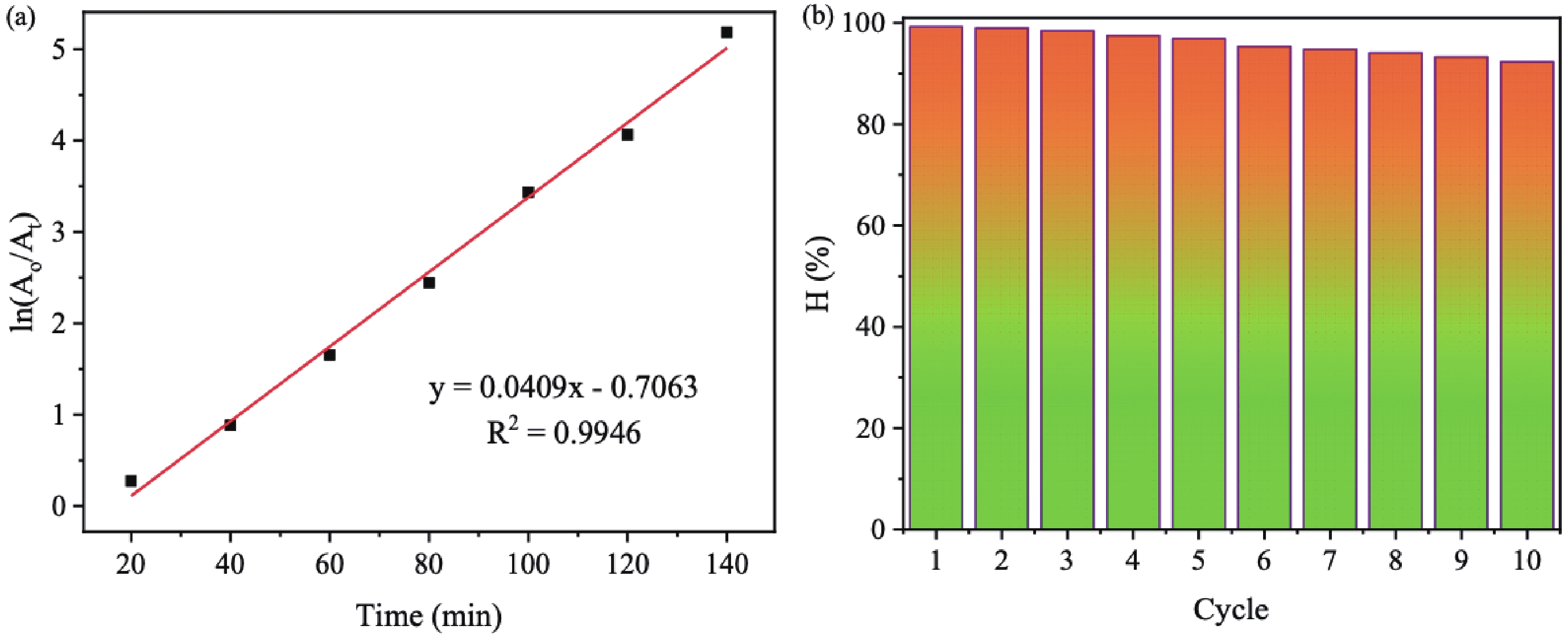
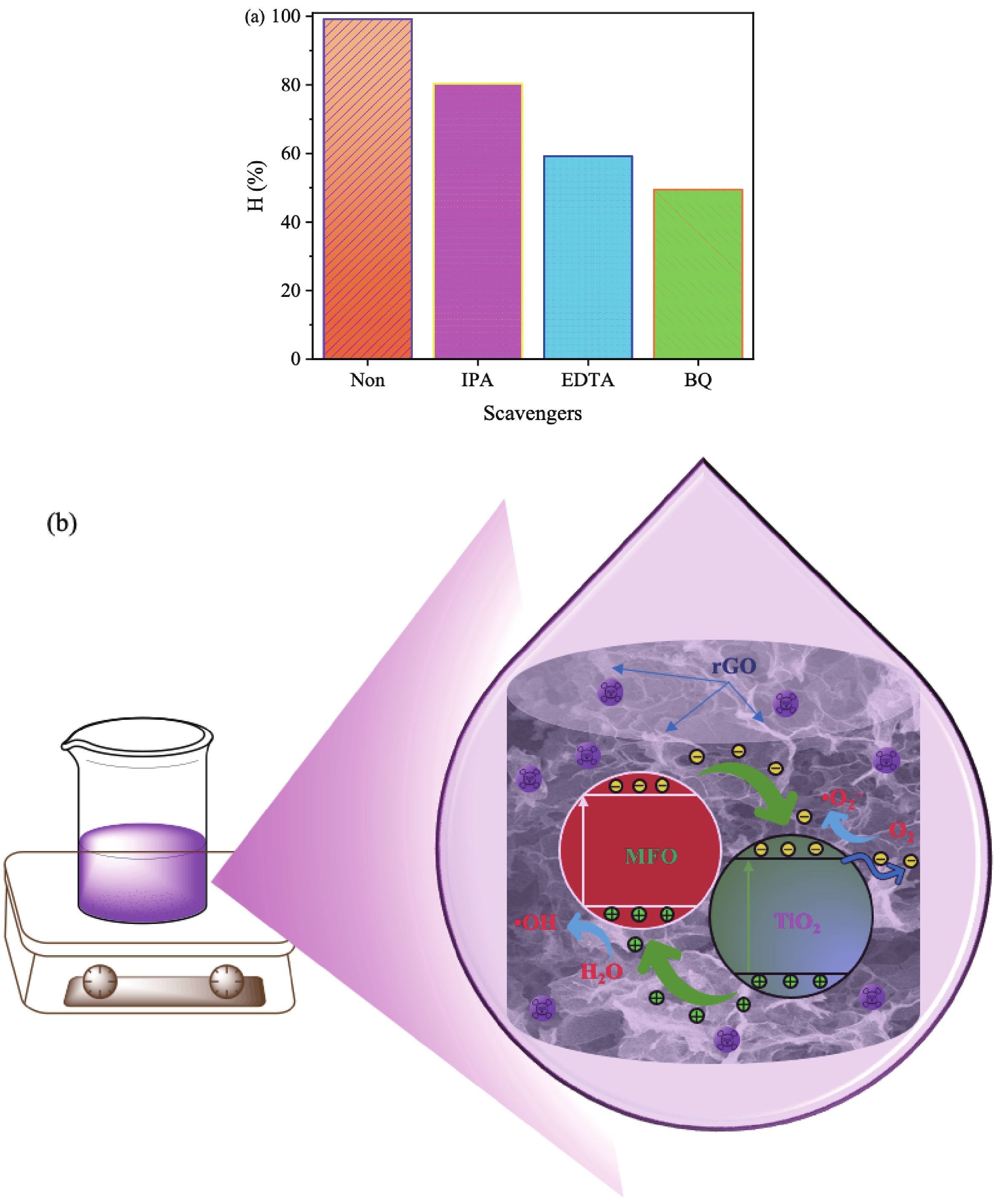
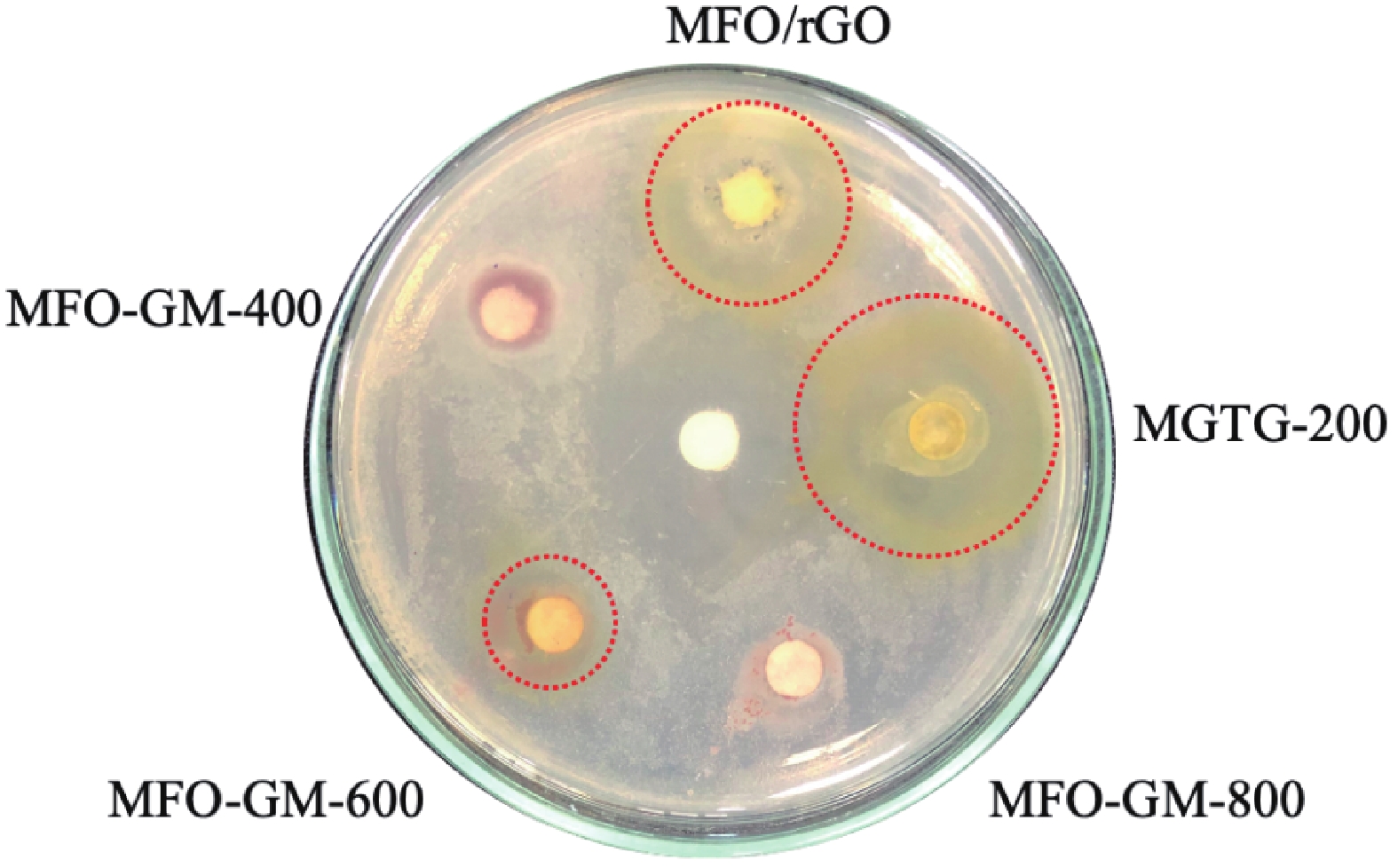
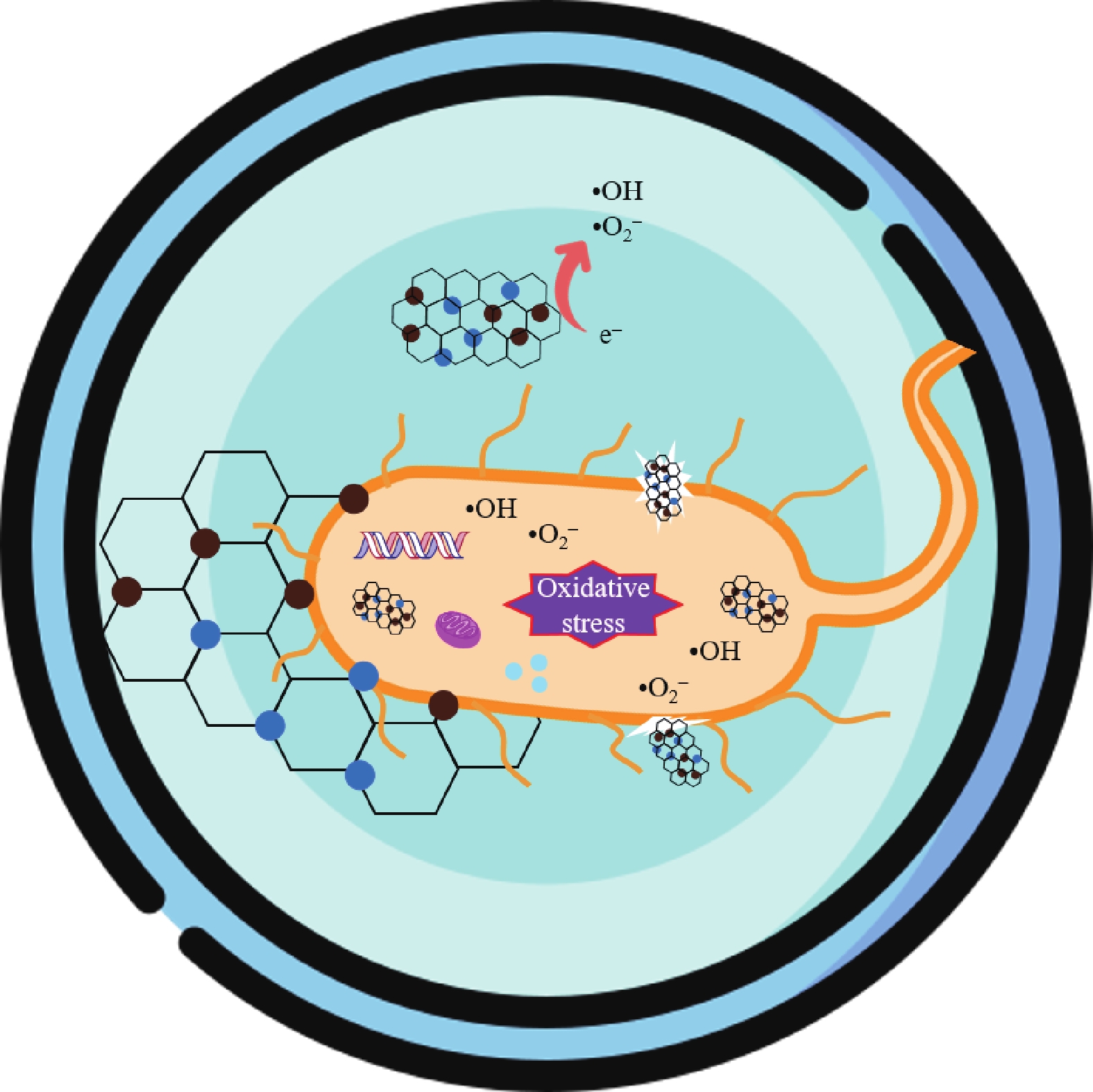
In this study, three-dimensional porous magnesium ferrite/titanium dioxide/reduced graphene oxide (MgFe2O4-GM/TiO2/rGO (MGTG)) was successfully synthesized via green and hydrothermal-supported co-precipitation methods using the extract of Garcinia mangostana (G. mangostana) as a reducing agent. The characterization results indicate the successful formation of the nano/micro MgFe2O4 (MFO) and TiO2 on the structure of the reduced graphene oxide (rGO), which can also act as efficient support, alleviating the agglomeration of the nano/micro MFO and TiO2. The synergic effects of the adsorption and photodegradation activity of the material were investigated according to the removal of crystal violet (CV) under ultraviolet light. The effects of catalyst dosage, CV concentration, and pH on the CV removal efficiency of the MGTG were also investigated. According to the results, the CV photodegradation of the MGTG-200 corresponded to the pseudo-first-order kinetic model. The reusability of the material after 10 cycles also showed a removal efficiency of 92%. This happened because the materials can easily be recollected using external magnets. In addition, according to the effects of different free radicals · O2−, h+, and · OH on the photodegradation process, the photocatalysis mechanism of the MGTG was also thoroughly suggested. The antibacterial efficiency of the MGTG was also evaluated according to the inhibition of the Gram-positive bacteria strain Staphylococcus aureus (S. aureus). Concurrently, the antibacterial mechanism of the fabricated material was also proposed. These results confirm that the prepared material can be potentially employed in a wide range of applications, including wastewater treatment and antibacterial activity. -
References
[1] Rajagopal S, Paramasivam B, Muniyasamy K. Photocatalytic removal of cationic and anionic dyes in the textile wastewater by H2O2 assisted TiO2 and micro-cellulose composites. Sep Purif Technol, 2020, 252, 117444 doi: 10.1016/j.seppur.2020.117444[2] Fatima B, Siddiqui S I, Rajor H K, et al. Photocatalytic removal of organic dye using green synthesized zinc oxide coupled cadmium tungstate nanocomposite under natural solar light irradiation. Environ Res, 2023, 216, 114534 doi: 10.1016/j.envres.2022.114534[3] Rahmat M, Rehman A, Rahmat S, et al. Highly efficient removal of crystal violet dye from water by MnO2 based nanofibrous mesh/photocatalytic process. J Mater Res Technol, 2019, 8, 5149 doi: 10.1016/j.jmrt.2019.08.038[4] Dargahi M, Masteri-Farahani M, Shahsavarifar S, et al. Microemulsion-mediated preparation of Ce2(MoO4)3 nanoparticles for photocatalytic degradation of crystal violet in aqueous solution. Environ Sci Pollut Res, 2020, 27, 12047 doi: 10.1007/s11356-020-07816-2[5] Sanakousar M F, Vidyasagar C C, Jiménez-Pérez V, et al. Efficient photocatalytic degradation of crystal violet dye and electrochemical performance of modified MWCNTs/Cd-ZnO nanoparticles with quantum chemical calculations. J Hazard Mater Adv, 2021, 2, 100004 doi: 10.1016/j.hazadv.2021.100004[6] Chen Y A, Xiang Z Y, Wang D S, et al. Effective photocatalytic degradation and physical adsorption of methylene blue using cellulose/GO/TiO2 hydrogels. RSC Adv, 2020, 10, 23936 doi: 10.1039/D0RA04509H[7] Spitaleri L, Nicotra G, Zimbone M, et al. Fast and efficient Sun light photocatalytic activity of Au_ZnO core−shell nanoparticles prepared by a one-pot synthesis. ACS Omega, 2019, 4, 15061 doi: 10.1021/acsomega.9b01850[8] Xie J A, Wen W, Jin Q, et al. TiO2 nanotrees for the photocatalytic and photoelectrocatalytic phenol degradation. New J Chem, 2019, 43, 11050 doi: 10.1039/C9NJ02219H[9] Chimupala Y, Phromma C, Yimklan S, et al. Dye wastewater treatment enabled by piezo-enhanced photocatalysis of single-component ZnO nanoparticles. RSC Adv, 2020, 10, 28567 doi: 10.1039/D0RA04746E[10] Zhang P, Xu J K, Wang X J, et al. The third generation of artificial dye-decolorizing peroxidase rationally designed in myoglobin. ACS Catal, 2019, 9, 7888 doi: 10.1021/acscatal.9b02226[11] Etman A S, Abdelhamid H N, Yuan Y Y, et al. Facile water-based strategy for synthesizing MoO3− x nanosheets: Efficient visible light photocatalysts for dye degradation. ACS Omega, 2018, 3, 2193 doi: 10.1021/acsomega.8b00012[12] Buu T T, Son V H, Nam N T H, et al. Three-dimensional ZnO-TiO2/graphene aerogel for water remediation: The screening studies of adsorption and photodegradation. Ceram Int, 2023, 49, 9868 doi: 10.1016/j.ceramint.2022.11.162[13] Uyguner Demirel C S, Birben N C, Bekbolet M. A comprehensive review on the use of second generation TiO2 photocatalysts: Microorganism inactivation. Chemosphere, 2018, 211, 420 doi: 10.1016/j.chemosphere.2018.07.121[14] Giang N T H, Huy L G, Khoi V H, et al. Enhanced photocatalytic activity of titanium dioxide-doped graphene aerogel towards p-nitrophenol removal from aqueous solutions. Mater Technol, 2022, 37, 2445 doi: 10.1080/10667857.2022.2038767[15] Yuan X Z, Wang H, Wu Y, et al. A novel SnS2-MgFe2O4/reduced graphene oxide flower-like photocatalyst: Solvothermal synthesis, characterization and improved visible-light photocatalytic activity. Catal Commun, 2015, 61, 62 doi: 10.1016/j.catcom.2014.12.003[16] Melia S, Novia D, Juliyarsi I, et al. The characteristics of the pericarp of garcinia mangostana (mangosteen) extract as natural antioxidants in rendang. IOP Conf Ser:Earth Environ Sci, 2019, 287, 012028 doi: 10.1088/1755-1315/287/1/012028[17] Apriandanu D O B, Yulizar Y. CuO-bentonite-gold nanocomposites: Facile green preparation and their characterization. Mater Lett, 2021, 284, 128911 doi: 10.1016/j.matlet.2020.128911[18] Sari I P, Yulizar Y. Green synthesis of magnetite (Fe3O4) nanoparticles using Graptophyllum pictum leaf aqueous extract. IOP Conf Ser:Mater Sci Eng, 2017, 191, 012014 doi: 10.1088/1757-899X/191/1/012014[19] Shahid M, Liu J L, Ali Z, et al. Photocatalytic degradation of methylene blue on magnetically separable MgFe2O4 under visible light irradiation. Mater Chem Phys, 2013, 139, 566 doi: 10.1016/j.matchemphys.2013.01.058[20] Tarcan R, Todor-Boer O, Petrovai I, et al. Reduced graphene oxide today. J Mater Chem C, 2020, 8, 1198 doi: 10.1039/C9TC04916A[21] Ye N, Wang Z, Wang S, et al. Aqueous aggregation and stability of graphene nanoplatelets, graphene oxide, and reduced graphene oxide in simulated natural environmental conditions: Complex roles of surface and solution chemistry. Environ Sci Pollut Res, 2018, 25, 10956 doi: 10.1007/s11356-018-1326-6[22] Jiao C L, Xiong J Q, Tao J, et al. Sodium alginate/graphene oxide aerogel with enhanced strength-toughness and its heavy metal adsorption study. Int J Biol Macromol, 2016, 83, 133 doi: 10.1016/j.ijbiomac.2015.11.061[23] Fu X G, Choi J Y, Zamani P, et al. Co−N decorated hierarchically porous graphene aerogel for efficient oxygen reduction reaction in acid. ACS Appl Mater Interfaces, 2016, 8, 6488 doi: 10.1021/acsami.5b12746[24] Long S X, Wang H, He K, et al. 3D graphene aerogel based photocatalysts: Synthesized, properties, and applications. Colloids Surf A, 2020, 594, 124666 doi: 10.1016/j.colsurfa.2020.124666[25] Viet T, Phong H, Thinh D B, et al. Enhanced photodegradation toward graphene−based MgFe2O4−TiO2: Investigation and optimization. Int J Hydrogen Energy, 2022, 47, 32092 doi: 10.1016/j.ijhydene.2022.07.119[26] Alkhouzaam A, Qiblawey H, Khraisheh M, et al. Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram Int, 2020, 46, 23997 doi: 10.1016/j.ceramint.2020.06.177[27] Duy P H A, Tu P M, Son T T, et al. A facile fabrication of zinc oxide-doped carbon aerogel by cellulose extracted from coconut peat and sodium alginate for energy storage application. J Appl Polym Sci, 2023, 140, e53837 doi: 10.1002/app.53837[28] Dat N M, Huong L M, Cong C Q, et al. Green synthesis of chitosan-based membrane modified with uniformly micro-sizing selenium particles decorated graphene oxide for antibacterial application. Int J Biol Macromol, 2022, 220, 348 doi: 10.1016/j.ijbiomac.2022.08.078[29] Ushakov M V, Nithya V D, Rajeesh Kumar N, et al. X-ray diffraction, magnetic measurements and Mössbauer spectroscopy of MgFe2O4 nanoparticles. J Alloys Compd, 2022, 912, 165125 doi: 10.1016/j.jallcom.2022.165125[30] Zhou L Y, Liu J X, Lu A M, et al. Controllable synthesis of cubic magnetic MgFe2O4 derived from MgFe-LDHs for efficient removal of methyl orange. Chem Eng J, 2022, 428, 131174 doi: 10.1016/j.cej.2021.131174[31] Zeeshan M, Yalcin K, Sarac Oztuna F E, et al. A new class of porous materials for efficient CO2 separation: Ionic liquid/graphene aerogel composites. Carbon, 2021, 171, 79 doi: 10.1016/j.carbon.2020.08.079[32] Israr M, Iqbal J, Arshad A, et al. Multifunctional MgFe2O4/GNPs nanocomposite: Graphene-promoted visible light driven photocatalytic activity and electrochemical performance of MgFe2O4 nanoparticles. Solid State Sci, 2020, 110, 106363 doi: 10.1016/j.solidstatesciences.2020.106363[33] Muwafaq M, Safa P. Hydrothermal synthesis and electrochemical performance of GNPs-doped MgFe2O4 electrodes for supercapacitors. Solid State Ion, 2023, 391, 116107 doi: 10.1016/j.ssi.2022.116107[34] Fu L M, Chen H W, Wang K, et al. Oxygen-vacancy generation in MgFe2O4 by high temperature calcination and its improved photocatalytic activity for CO2 reduction. J Alloys Compd, 2022, 891, 161925 doi: 10.1016/j.jallcom.2021.161925[35] Kumar G M, Cho H D, Lee D J, et al. Elevating the charge separation of MgFe2O4 nanostructures by Zn ions for enhanced photocatalytic and photoelectrochemical water splitting. Chemosphere, 2021, 283, 131134 doi: 10.1016/j.chemosphere.2021.131134[36] Tiano A L, Papaefthymiou G C, Lewis C S, et al. Correlating size and composition-dependent effects with magnetic, mössbauer, and pair distribution function measurements in a family of catalytically active ferrite nanoparticles. Chem Mater, 2015, 27, 3572 doi: 10.1021/acs.chemmater.5b00767[37] Zu Y Q, Zhao Y Q, Xu K Z, et al. Preparation and comparison of catalytic performance for nano MgFe2O4, GO-loaded MgFe2O4 and GO-coated MgFe2O4 nanocomposites. Ceram Int, 2016, 42, 18844 doi: 10.1016/j.ceramint.2016.09.030[38] Li J N, Yu Y L, Chen D H, et al. Hydrophilic graphene aerogel anodes enhance the performance of microbial electrochemical systems. Bioresour Technol, 2020, 304, 122907 doi: 10.1016/j.biortech.2020.122907[39] Shakir I, Sarfraz M, Ali Z, et al. Magnetically separable and recyclable graphene-MgFe2O4 nanocomposites for enhanced photocatalytic applications. J Alloys Compd, 2016, 660, 450 doi: 10.1016/j.jallcom.2015.11.055[40] Da Silva E P, Guilherme M R, Tenório-Neto E T, et al. scCO2-based synthesis of semi-crystalline TiO2 nanoparticles: A rapid and direct strategy. Mater Lett, 2014, 136, 133 doi: 10.1016/j.matlet.2014.07.156[41] Guerrero-Urbaneja P, García-Sancho C, Moreno-Tost R, et al. Glycerol valorization by etherification to polyglycerols by using metal oxides derived from MgFe hydrotalcites. Appl Catal A, 2014, 470, 199 doi: 10.1016/j.apcata.2013.10.051[42] Jadhav S V, Kim B M, Lee H Y, et al. Induction heating and in vitro cytotoxicity studies of MnZnFe2O4 nanoparticles for self-controlled magnetic particle hyperthermia. J Alloys Compd, 2018, 745, 282 doi: 10.1016/j.jallcom.2018.02.174[43] Tang S Q, Moon S J, Park K H, et al. Feasibility of TEOS coated CoFe2O4 nanoparticles to a GMR biosensor agent for single molecular detection. J Nanosci Nanotechnol, 2011, 11, 82 doi: 10.1166/jnn.2011.3043[44] Baskoro F, Wong C B, Kumar S R, et al. Graphene oxide-cation interaction: Inter-layer spacing and zeta potential changes in response to various salt solutions. J Membr Sci, 2018, 554, 253 doi: 10.1016/j.memsci.2018.03.006[45] Vusa C S R, Berchmans S, Alwarappan S. Facile and green synthesis of graphene. RSC Adv, 2014, 4, 22470 doi: 10.1039/C4RA01718H[46] Yan J, Liu J P, Fan Z J, et al. High-performance supercapacitor electrodes based on highly corrugated graphene sheets. Carbon, 2012, 50, 2179 doi: 10.1016/j.carbon.2012.01.028[47] Huy B T, Jung D S, Kim Phuong N T, et al. Enhanced photodegradation of 2, 4-dichlorophenoxyacetic acid using a novel TiO2@MgFe2O4 core@shell structure. Chemosphere, 2017, 184, 849 doi: 10.1016/j.chemosphere.2017.06.069[48] Dat N M, Cong C Q, Phuc N M, et al. Facile phytosynthesis of gold nanoparticles-doped graphene oxide using Mangifera indica leaf extract: Characterization, antibacterial activity, and catalytic reduction of organic dyes. Mater Today Sustain, 2022, 19, 100216 doi: 10.1016/j.mtsust.2022.100216[49] Feng Q, Li S Y, Ma W H, et al. Synthesis and characterization of Fe3O4/ZnO-GO nanocomposites with improved photocatalytic degradation methyl orange under visible light irradiation. J Alloys Compd, 2018, 737, 197 doi: 10.1016/j.jallcom.2017.12.070[50] Alharthi F A, Ali Alghamdi A, Al-Zaqri N, et al. Facile one-pot green synthesis of Ag−ZnO Nanocomposites using potato peeland their Ag concentration dependent photocatalytic properties. Sci Rep, 2020, 10, 20229 doi: 10.1038/s41598-020-77426-y[51] Kazeminezhad I, Sadollahkhani A. Influence of pH on the photocatalytic activity of ZnO nanoparticles. J Mater Sci: Mater Electron, 2016, 27, 4206 doi: 10.1007/s10854-016-4284-0[52] Zhang D F, Zeng F B. Visible light-activated cadmium-doped ZnO nanostructured photocatalyst for the treatment of methylene blue dye. J Mater Sci, 2012, 47, 2155 doi: 10.1007/s10853-011-6016-4[53] Kumar A. A review on the factors affecting the photocatalytic degradation of hazardous materials. Mater Sci Eng Int J, 2017, 1 doi: 10.15406/mseij.2017.01.00018[54] Lu P, Hu X L, Li Y J, et al. Novel CaCO3/g-C3N4 composites with enhanced charge separation and photocatalytic activity. J Saudi Chem Soc, 2019, 23, 1109 doi: 10.1016/j.jscs.2019.07.002[55] Mohamed S K, Hegazy S H, Abdelwahab N A, et al. Coupled adsorption-photocatalytic degradation of crystal violet under sunlight using chemically synthesized grafted sodium alginate/ZnO/graphene oxide composite. Int J Biol Macromol, 2018, 108, 1185 doi: 10.1016/j.ijbiomac.2017.11.028[56] Ajiboye T O, Oyewo O A, Marzouki R, et al. Synthesis of AgBiS2/gC3N4 and its application in the photocatalytic reduction of Pb(II) in the matrix of methyl orange, crystal violet, and methylene blue dyes. Ceram Int, 2023, 49, 6149 doi: 10.1016/j.ceramint.2022.10.187[57] Zhang H, He J, Wu P, et al. Facile synthesis of Z-scheme KBiO3/g-C3N4 Z-scheme heterojunction photocatalysts: Structure, performance, and mechanism. J Environ Chem Eng, 2022, 10, 107804 doi: 10.1016/j.jece.2022.107804[58] Kaur J, Kaur M. Facile fabrication of ternary nanocomposite of MgFe2O4 TiO2@GO for synergistic adsorption and photocatalytic degradation studies. Ceram Int, 2019, 45, 8646 doi: 10.1016/j.ceramint.2019.01.185[59] Guzel Kaya G, Aznar E, Deveci H, et al. Aerogels as promising materials for antibacterial applications: A mini-review. Biomater Sci, 2021, 9, 7034 doi: 10.1039/D1BM01147B[60] Vargas M A, Rodríguez-Páez J E. Amorphous TiO2 nanoparticles: Synthesis and antibacterial capacity. J Non Cryst Solids, 2017, 459, 192 doi: 10.1016/j.jnoncrysol.2017.01.018[61] Priya R S, Kumar E R, Balamurugan A, et al. Green synthesized MgFe2O4 ferrites nanoparticles for biomedical applications. Appl Phys A, 2021, 127, 538 doi: 10.1007/s00339-021-04699-z[62] Chandrasekaran M, Kim K D, Chun S C. Antibacterial activity of chitosan nanoparticles: A review. Processes, 2020, 8, 1173 doi: 10.3390/pr8091173[63] Hashem A H, Khalil A M A, Reyad A M, et al. Biomedical applications of mycosynthesized selenium nanoparticles using penicillium expansum ATTC 36200. Biol Trace Elem Res, 2021, 199, 3998 doi: 10.1007/s12011-020-02506-z[64] Khan A U, Arooj A, Tahir K, et al. Facile fabrication of novel Ag2S-ZnO/GO nanocomposite with its enhanced photocatalytic and biological applications. J Mol Struct, 2022, 1251, 131991 doi: 10.1016/j.molstruc.2021.131991[65] Shoran S, Chaudhary S, Sharma A. Photocatalytic dye degradation and antibacterial activities of CeO2/g-C3N4 nanomaterials for environmental applications. Environ Sci Pollut Res, 2023, 30, 98682 doi: 10.1007/s11356-022-23815-x[66] Dineshbabu N, Jayaprakash R N, Karuppasamy P, et al. Investigation on Tetracycline degradation and bactericidal properties of binary and ternary ZnO/NiO/g-C3N4 composites prepared by a facile co-precipitation method. J Environ Chem Eng, 2022, 10, 107368 doi: 10.1016/j.jece.2022.107368[67] Othman N H, Alias N H, Shahruddin M Z, et al. Adsorption kinetics of methylene blue dyes onto magnetic graphene oxide. J Environ Chem Eng, 2018, 6, 2803 doi: 10.1016/j.jece.2018.04.024[68] Cacaci M, Martini C, Guarino C, et al. Graphene oxide coatings as tools to prevent microbial biofilm formation on medical device. Advances in Experimental Medicine and Biology. Springer International Publishing, 2019, 14, 21 doi: 10.1007/5584_2019_434 -
Proportional views





 DownLoad:
DownLoad:










 Tong Hoang Lin received her Bachelor’s degree in 2020 from Ho Chi Minh City University of Industry and Trade and her Master’s degree in 2023 from Ho Chi Minh City University of Technology under the supervision of Assoc. Prof. Nguyen Huu Hieu. Her research focuses on graphene photocatalytic materials
Tong Hoang Lin received her Bachelor’s degree in 2020 from Ho Chi Minh City University of Industry and Trade and her Master’s degree in 2023 from Ho Chi Minh City University of Technology under the supervision of Assoc. Prof. Nguyen Huu Hieu. Her research focuses on graphene photocatalytic materials Che Quang Cong is currently studying and conducting research at the Key CEPP Laboratory in Chemical Engineering in Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City under the supervision of Assoc. Prof. Nguyen Huu Hieu. His research interests include nanomaterials in photocatalytic application using bio-mediation methodology
Che Quang Cong is currently studying and conducting research at the Key CEPP Laboratory in Chemical Engineering in Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City under the supervision of Assoc. Prof. Nguyen Huu Hieu. His research interests include nanomaterials in photocatalytic application using bio-mediation methodology Hoang An recently received his Bachelor’s degree in chemical engineering from the Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City and currently pursues a Master’s degree in the same institution under the supervision of Assoc. Prof. Nguyen Huu Hieu. His research interests include photocatalyst and composite material, and graphitic carbon nitride-based material
Hoang An recently received his Bachelor’s degree in chemical engineering from the Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City and currently pursues a Master’s degree in the same institution under the supervision of Assoc. Prof. Nguyen Huu Hieu. His research interests include photocatalyst and composite material, and graphitic carbon nitride-based material Nguyen Duy Hai recently received his Bachelor’s degree in chemical engineering from the Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City and is currently pursuing a Master’s degree in the same institution under the supervision of Assoc. Prof. Nguyen Huu Hieu. His research interests include the fabrication of graphene-based nanocomposites for the application in catalysis, colorimetric detection, and antimicrobial activities
Nguyen Duy Hai recently received his Bachelor’s degree in chemical engineering from the Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City and is currently pursuing a Master’s degree in the same institution under the supervision of Assoc. Prof. Nguyen Huu Hieu. His research interests include the fabrication of graphene-based nanocomposites for the application in catalysis, colorimetric detection, and antimicrobial activities Ton That Buu received his Bachelor’s degree in 2021 from University of Science, Viet Nam National University Ho Chi Minh City and his Master’s degree in 2023 from University of Technology under the supervision of Assoc. Prof. Nguyen Huu Hieu. His research focuses on graphene, photocatalytic materials
Ton That Buu received his Bachelor’s degree in 2021 from University of Science, Viet Nam National University Ho Chi Minh City and his Master’s degree in 2023 from University of Technology under the supervision of Assoc. Prof. Nguyen Huu Hieu. His research focuses on graphene, photocatalytic materials Lam Thanh Ngan is currently studying and conducting research at the Key CEPP Laboratory in Chemical Engineering at Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City under the supervision of Assoc. Prof. Nguyen Huu Hieu. Her research interests include nanomaterials in photocatalytic application
Lam Thanh Ngan is currently studying and conducting research at the Key CEPP Laboratory in Chemical Engineering at Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City under the supervision of Assoc. Prof. Nguyen Huu Hieu. Her research interests include nanomaterials in photocatalytic application Hoang Thuy Kim Ngan is currently studying and conducting research at the Key CEPP Laboratory in Chemical Engineering at Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City under the supervision of Assoc. Prof. Nguyen Huu Hieu. Her research interests include nanomaterials in photocatalytic application
Hoang Thuy Kim Ngan is currently studying and conducting research at the Key CEPP Laboratory in Chemical Engineering at Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City under the supervision of Assoc. Prof. Nguyen Huu Hieu. Her research interests include nanomaterials in photocatalytic application Ta Dang Khoa received his doctoral degree from the Chemical Engineering Department, Universiti Teknologi PETRONAS, in 2012. He is currently associate professor in chemical engineering in Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City. His current research interests include adsorption materials and photodegradation materials
Ta Dang Khoa received his doctoral degree from the Chemical Engineering Department, Universiti Teknologi PETRONAS, in 2012. He is currently associate professor in chemical engineering in Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City. His current research interests include adsorption materials and photodegradation materials Nguyen Huu Hieu received his doctoral degree from Chonbuk National University, Korea, in 2012. He is currently associate professor in chemical engineering in Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City. His current research interests include graphene aerogel, adsorption materials, photodegradation materials
Nguyen Huu Hieu received his doctoral degree from Chonbuk National University, Korea, in 2012. He is currently associate professor in chemical engineering in Ho Chi Minh University of Technology, Viet Nam National University Ho Chi Minh City. His current research interests include graphene aerogel, adsorption materials, photodegradation materials



