| Citation: |
Xiaofei Liu, Songyin Qiu, Haiping Fang, Lin Mei, Hongli Jing, Chunyan Feng, Shaoqiang Wu, Xiangmei Lin. A brief review of novel nucleic acid test biosensors and their application prospects for salmonids viral diseases detection[J]. Journal of Semiconductors, 2023, 44(2): 023103. doi: 10.1088/1674-4926/44/2/023103
****
X F Liu, S Y Qiu, H P Fang, L Mei, H L Jing, C Y Feng, S Q Wu, X M Lin. A brief review of novel nucleic acid test biosensors and their application prospects for salmonids viral diseases detection[J]. J. Semicond, 2023, 44(2): 023103. doi: 10.1088/1674-4926/44/2/023103
|
A brief review of novel nucleic acid test biosensors and their application prospects for salmonids viral diseases detection
DOI: 10.1088/1674-4926/44/2/023103
More Information
-
Abstract
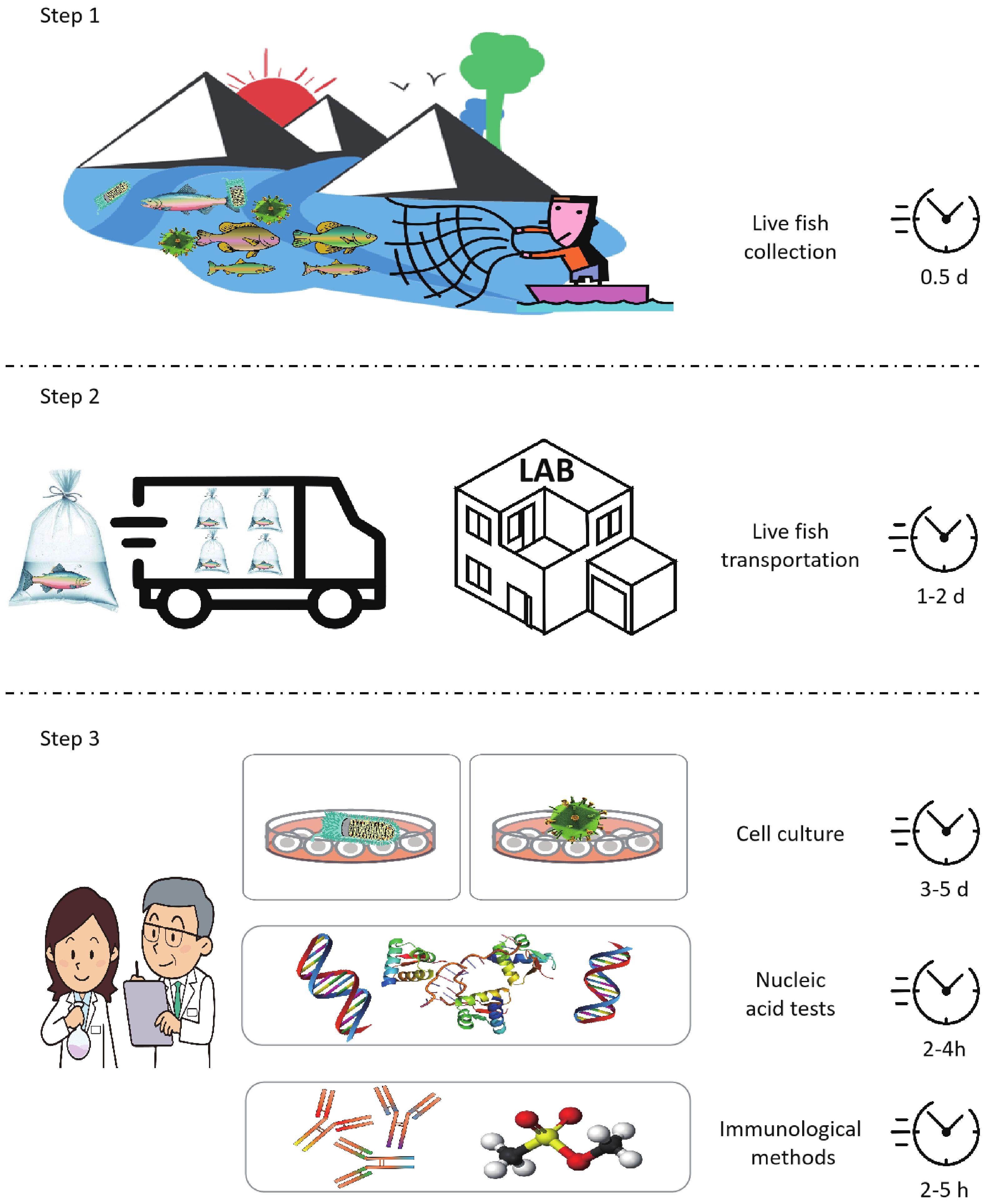
Viral diseases represent one of the major threats for salmonids aquaculture. Early detection and identification of viral pathogens is the main prerequisite prior to undertaking effective prevention and control measures. Rapid, sensitive, efficient and portable detection method is highly essential for fish viral diseases detection. Biosensor strategies are highly prevalent and fulfill the expanding demands of on-site detection with fast response, cost-effectiveness, high sensitivity, and selectivity. With the development of material science, the nucleic acid biosensors fabricated by semiconductor have shown great potential in rapid and early detection or screening for diseases at salmonids fisheries. This paper reviews the current detection development of salmonids viral diseases. The present limitations and challenges of salmonids virus diseases surveillance and early detection are presented. Novel nucleic acid semiconductor biosensors are briefly reviewed. The perspective and potential application of biosensors in the on-site detection of salmonids diseases are discussed.-
Keywords:
- salmonids virus,
- detection,
- nucleic acid test,
- biosensors,
- semiconductor
-
References
[1] Bianchi M C, Chopin F, Farme T, et al. FAO: the state of world fisheries and aquaculture. Rome, Italy: Food and Agriculture Organization of the United Nations, 2014[2] Crane M, Hyatt A. Viruses of fish: An overview of significant pathogens. Viruses, 2011, 3, 2025 doi: 10.3390/v3112025[3] Pinheiro A C A S, Volpe E, Principi D, et al. Development of a multiplex RT-PCR assay for simultaneous detection of the major viruses that affect rainbow trout (Oncorhynchus mykiss). Aquacult Int, 2016, 24, 115 doi: 10.1007/s10499-015-9912-9[4] Rodriguez Saint-Jean S, Borrego J J, Perez-Prieto S I. Comparative evaluation of five serological methods and RT-PCR assay for the detection of IPNV in fish. J Virol Methods, 2001, 97, 23 doi: 10.1016/S0166-0934(01)00329-9[5] Hoferer M, Akimkin V, Skrypski J, et al. Improvement of a diagnostic procedure in surveillance of the listed fish diseases IHN and VHS. J Fish Dis, 2019, 42, 559 doi: 10.1111/jfd.12968[6] Shao Y Z, Zhao J Z, Ren G M, et al. Early or simultaneous infection with infectious pancreatic necrosis virus inhibits infectious hematopoietic necrosis virus replication and induces a stronger antiviral response during Co-infection in rainbow trout (Oncorhynchus mykiss). Viruses, 2022, 14, 1732 doi: 10.3390/v14081732[7] Menon S, Mathew M R, Sam S, et al. Recent advances and challenges in electrochemical biosensors for emerging and re-emerging infectious diseases. J Electroanal Chem, 2020, 878, 114596 doi: 10.1016/j.jelechem.2020.114596[8] Bhalla N, Jolly P, Formisano N, et al. Introduction to biosensors. Essays Biochem, 2016, 60, 1 doi: 10.1042/EBC20150001[9] Kim U, Ghanbari S, Ravikumar A, et al. Rapid, affordable, and point-of-care water monitoring via a microfluidic DNA sensor and a mobile interface for global health. IEEE J Transl Eng Health Med, 2013, 1, 3700207 doi: 10.1109/JTEHM.2013.2281819[10] Gavrilescu M, Demnerová K, Aamand J, et al. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol, 2015, 32, 147 doi: 10.1016/j.nbt.2014.01.001[11] Chen Y, Wang Z Z, Liu Y X, et al. Recent advances in rapid pathogen detection method based on biosensors. Eur J Clin Microbiol Infect Dis, 2018, 37, 1021 doi: 10.1007/s10096-018-3230-x[12] Pandey C M, Augustine S, Kumar S, et al. Microfluidics based point-of-care diagnostics. Biotechnol J, 2018, 13, 1700047 doi: 10.1002/biot.201700047[13] Amar E C, Kiron V, Akutsu T, et al. Resistance of rainbow trout Oncorhynchus mykiss to infectious hematopoietic necrosis virus (IHNV) experimental infection following ingestion of natural and synthetic carotenoids. Aquaculture, 2012, 330, 148 doi: 10.1016/j.aquaculture.2011.12.007[14] Ahmadivand S, Soltani M, Mardani K, et al. Infectious hematopoietic necrosis virus (IHNV) outbreak in farmed rainbow trout in Iran: Viral isolation, pathological findings, molecular confirmation, and genetic analysis. Virus Res, 2017, 229, 17 doi: 10.1016/j.virusres.2016.12.013[15] WOAH. Infection with infectious haematopoietic necrosis virus. Manual of Diagnostic Tests for Aquatic Animals, 2021, chapter 2.3.5[16] Ziafati Kafi Z, Ghalyanchilangeroudi A, Nikaein D, et al. Phylogenetic analysis and genotyping of Iranian infectious haematopoietic necrosis virus (IHNV) of rainbow trout (Oncorhynchus mykiss) based on the glycoprotein gene. Vet Med Sci, 2022, 8, 2411 doi: 10.1002/vms3.931[17] Kurath G, Winton J R, Dale O B, et al. Atlantic salmon, Salmo salar L. are broadly susceptible to isolates representing the North American genogroups of infectious hematopoietic necrosis virus. J Fish Dis, 2016, 39, 55 doi: 10.1111/jfd.12323[18] Tapia D, Eissler Y, Torres P, et al. Detection and phylogenetic analysis of infectious pancreatic necrosis virus in Chile. Dis Aquat Organ, 2015, 116, 173 doi: 10.3354/dao02912[19] Zhu L, Wang X L, Wang K Y, et al. Outbreak of infectious pancreatic necrosis virus (IPNV) in farmed rainbow trout in China. Acta Trop, 2017, 170, 63 doi: 10.1016/j.actatropica.2017.02.025[20] Suebsing R, Oh M J, Kim J H. Evaluation of rapid and sensitive reverse transcription loop-mediated isothermal amplification method for detecting infectious pancreatic necrosis virus in chum salmon (Oncorhynchus Keta). J Vet Diagn Invest, 2011, 23, 704 doi: 10.1177/1040638711407897[21] Yong C Y, Ong H K, Tang H C, et al. Infectious hematopoietic necrosis virus: Advances in diagnosis and vaccine development. PeerJ, 2019, 7, e7151 doi: 10.7717/peerj.7151[22] Ning B, Huang Z, Youngquist B M, et al. Liposome-mediated detection of SARS-CoV-2 RNA-positive extracellular vesicles in plasma. Nat Nanotechnol, 2021, 16, 1039 doi: 10.1038/s41565-021-00939-8[23] Cella L N, Blackstock D, Yates M A, et al. Detection of RNA viruses: Current technologies and future perspectives. Crit Rev Eukaryot Gene Expr, 2013, 23, 125 doi: 10.1615/CritRevEukaryotGeneExpr.2013006974[24] Dhar A K, Bowers R M, Licon K S, et al. Detection and quantification of infectious hematopoietic necrosis virus in rainbow trout (Oncorhynchus mykiss) by SYBR Green real-time reverse transcriptase-polymerase chain reaction. J Virol Methods, 2008, 147, 157 doi: 10.1016/j.jviromet.2007.08.026[25] Liu Z X, Teng Y, Liu H, et al. Simultaneous detection of three fish rhabdoviruses using multiplex real-time quantitative RT-PCR assay. J Virol Methods, 2008, 149, 103 doi: 10.1016/j.jviromet.2007.12.017[26] Purcell M K, Thompson R L, Garver K A, et al. Universal reverse-transcriptase real-time PCR for infectious hematopoietic necrosis virus (IHNV). Dis Aquat Organ, 2013, 106, 103 doi: 10.3354/dao02644[27] Kerr C C, Cunningham C O. Moving molecular diagnostics from laboratory to clinical application: A case study using infectious pancreatic necrosis virus serotype A. Lett Appl Microbiol, 2006, 43, 98 doi: 10.1111/j.1472-765X.2006.01885.x[28] Alonso M, Rodríguez S, Pérez-Prieto S I. Viral coinfection in salmonids: Infectious pancreatic necrosis virus interferes with infectious hematopoietic necrosis virus. Arch Virol, 1999, 144, 657 doi: 10.1007/s007050050534[29] Hoferer M, Braun A, Skrypski J, et al. One-step cross-genogroup multiplex RT-qPCR with an internal control system for the detection of infectious pancreatic necrosis virus (IPNV). J Virol Methods, 2017, 247, 68 doi: 10.1016/j.jviromet.2017.05.015[30] Orpetveit I, Mikalsen A B, Sindre H, et al. Detection of infectious pancreatic necrosis virus in subclinically infected Atlantic salmon by virus isolation in cell culture or real-time reverse transcription polymerase chain reaction: Influence of sample preservation and storage. J Vet Diagn Invest, 2010, 22, 886 doi: 10.1177/104063871002200606[31] Bowers R M, Lapatra S E, Dhar A K. Detection and quantitation of infectious pancreatic necrosis virus by real-time reverse transcriptase-polymerase chain reaction using lethal and non-lethal tissue sampling. J Virol Methods, 2008, 147, 226 doi: 10.1016/j.jviromet.2007.09.003[32] Jia P, Purcell M K, Pan G, et al. Analytical validation of a reverse transcriptase droplet digital PCR (RT-ddPCR) for quantitative detection of infectious hematopoietic necrosis virus. J Virol Methods, 2017, 245, 73 doi: 10.1016/j.jviromet.2017.03.010[33] Cuenca A, Vendramin N, Olesen N J. Analytical validation of one-step realtime RT-PCR for detection of infectious hematopoietic necrosis virus (IHNV). Bull Eur Ass Fish Pathol, 2020, 40, 261[34] Suebsing R, Kim J H, Kim S R, et al. Detection of viruses in farmed rainbow trout (Oncorhynchus mykiss) in Korea by RT-LAMP assay. J Microbiol, 2011, 49, 741 doi: 10.1007/s12275-011-1209-8[35] Suebsing R, Jeon C H, Oh M J, et al. Reverse transcriptase loop-mediated isothermal amplification assay for infectious hematopoietic necrosis virus in Oncorhynchus Keta. Dis Aquat Organ, 2011, 94, 1 doi: 10.3354/dao02310[36] Gunimaladevi I, Kono T, Lapatra S E, et al. A loop mediated isothermal amplification (LAMP) method for detection of infectious hematopoietic necrosis virus (IHNV) in rainbow trout (Oncorhynchus mykiss). Arch Virol, 2005, 150, 899 doi: 10.1007/s00705-004-0468-7[37] Soliman H, Midtlyng P J, El-Matbouli M. Sensitive and rapid detection of infectious pancreatic necrosis virus by reverse transcription loop mediated isothermal amplification. J Virol Methods, 2009, 158, 77 doi: 10.1016/j.jviromet.2009.01.018[38] Zambry N S, Obande G A, Khalid M F, et al. Utilizing electrochemical-based sensing approaches for the detection of SARS-CoV-2 in clinical samples: A review. Biosensors, 2022, 12, 473 doi: 10.3390/bios12070473[39] Chen H, Liu K K, Li Z, et al. Point of care testing for infectious diseases. Clin Chimica Acta, 2019, 493, 138 doi: 10.1016/j.cca.2019.03.008[40] Zang Y, Fan J, Ju Y, et al. Current advances in semiconductor nanomaterial-based photoelectrochemical biosensing. Chemistry, 2018, 24, 14010 doi: 10.1002/chem.201801358[41] Tay A, Pavesi A, Yazdi S R, et al. Advances in microfluidics in combating infectious diseases. Biotechnol Adv, 2016, 34, 404 doi: 10.1016/j.biotechadv.2016.02.002[42] Escobar A, Chiu P, Qu J X, et al. Integrated microfluidic-based platforms for on-site detection and quantification of infectious pathogens: Towards on-site medical translation of SARS-CoV-2 diagnostic platforms. Micromachines, 2021, 12, 1079 doi: 10.3390/mi12091079[43] Zhang L, Ding B Z, Chen Q H, et al. Point-of-care-testing of nucleic acids by microfluidics. Trac Trends Anal Chem, 2017, 94, 106 doi: 10.1016/j.trac.2017.07.013[44] Jagannath A, Cong H J, Hassan J, et al. Pathogen detection on microfluidic platforms: Recent advances, challenges, and prospects. Biosens Bioelectron X, 2022, 10, 100134 doi: 10.1016/j.biosx.2022.100134[45] Wu Q Y, Zhang Y Z, Yang Q, et al. Review of electrochemical DNA biosensors for detecting food borne pathogens. Sensors, 2019, 19, 4916 doi: 10.3390/s19224916[46] Maffert P, Reverchon S, Nasser W, et al. New nucleic acid testing devices to diagnose infectious diseases in resource-limited settings. Eur J Clin Microbiol Infect Dis, 2017, 36, 1717 doi: 10.1007/s10096-017-3013-9[47] Jie J Y, Hu S M, Liu W W, et al. Portable and battery-powered PCR device for DNA amplification and fluorescence detection. Sensors, 2020, 20, 2627 doi: 10.3390/s20092627[48] Sun F, Ganguli A, Nguyen J, et al. Smartphone-based multiplex 30-minute nucleic acid test of live virus from nasal swab extract. Lab Chip, 2020, 20, 1621 doi: 10.1039/D0LC00304B[49] Powell L, Wiederkehr R S, Damascus P, et al. Rapid and sensitive detection of viral nucleic acids using silicon microchips. Analyst, 2018, 143, 2596 doi: 10.1039/C8AN00552D[50] Nunez-Bajo E, Silva Pinto Collins A, Kasimatis M, et al. Disposable silicon-based all-in-one micro-qPCR for rapid on-site detection of pathogens. Nat Commun, 2020, 11, 6176 doi: 10.1038/s41467-020-19911-6[51] Blair E O, Corrigan D K. A review of microfabricated electrochemical biosensors for DNA detection. Biosens Bioelectron, 2019, 134, 57 doi: 10.1016/j.bios.2019.03.055[52] Barreda-García S, Miranda-Castro R, de-Los-Santos-Álvarez N, et al. Solid-phase helicase dependent amplification and electrochemical detection of Salmonella on highly stable oligonucleotide-modified ITO electrodes. Chem Commun, 2017, 53, 9721 doi: 10.1039/C7CC05128J[53] Barreda-García S, Miranda-Castro R, de-los-Santos-Álvarez N, et al. Sequence-specific electrochemical detection of enzymatic amplification products of Salmonella genome on ITO electrodes improves pathogen detection to the single copy level. Sens Actuat B, 2018, 268, 438 doi: 10.1016/j.snb.2018.04.133[54] Zang Y, Lei J P, Hao Q, et al. CdS/MoS2 heterojunction-based photoelectrochemical DNA biosensor via enhanced chemiluminescence excitation. Biosens Bioelectron, 2016, 77, 557 doi: 10.1016/j.bios.2015.10.010[55] Asal M, Özen Ö, Şahinler M, et al. Recent developments in enzyme, DNA and immuno-based biosensors. Sensors, 2018, 18, 1924 doi: 10.3390/s18061924[56] Li Y Q, Sun L, Liu Q, et al. Photoelectrochemical CaMV35S biosensor for discriminating transgenic from non-transgenic soybean based on SiO2@CdTe quantum dots core-shell nanoparticles as signal indicators. Talanta, 2016, 161, 211 doi: 10.1016/j.talanta.2016.08.047[57] Shu J, Qiu Z L, Lv S Z, et al. Plasmonic enhancement coupling with defect-engineered TiO2–x: A mode for sensitive photoelectrochemical biosensing. Anal Chem, 2018, 90, 2425 doi: 10.1021/acs.analchem.7b05296[58] Zhu C F, Zeng Z Y, Li H, et al. Single-layer MoS2-based nanoprobes for homogeneous detection of biomolecules. J Am Chem Soc, 2013, 135, 5998 doi: 10.1021/ja4019572[59] Wang X X, Nan F X, Zhao J L, et al. A label-free ultrasensitive electrochemical DNA sensor based on thin-layer MoS2 nanosheets with high electrochemical activity. Biosens Bioelectron, 2015, 64, 386 doi: 10.1016/j.bios.2014.09.030[60] Ma X G, Wang C, Wu F X, et al. TiO2 nanomaterials in photoelectrochemical and electrochemiluminescent biosensing. Top Curr Chem, 2020, 378, 28 doi: 10.1007/s41061-020-0291-y[61] Liu X P, Chen J S, Mao C J, et al. Enhanced photoelectrochemical DNA sensor based on TiO2/Au hybrid structure. Biosens Bioelectron, 2018, 116, 23 doi: 10.1016/j.bios.2018.05.036[62] Chen C C, Lai Z L, Wang G J, et al. Polymerase chain reaction-free detection of hepatitis B virus DNA using a nanostructured impedance biosensor. Biosens Bioelectron, 2016, 77, 603 doi: 10.1016/j.bios.2015.10.028[63] Zhu H T, Wang J X, Xu G Y. Fast synthesis of Cu2O hollow microspheres and their application in DNA biosensor of hepatitis B virus. Cryst Growth Des, 2009, 9, 633 doi: 10.1021/cg801006g[64] Shariati M. The field effect transistor DNA biosensor based on ITO nanowires in label-free hepatitis B virus detecting compatible with CMOS technology. Biosens Bioelectron, 2018, 105, 58 doi: 10.1016/j.bios.2018.01.022[65] Shariati M, Sadeghi M. Ultrasensitive DNA biosensor for hepatitis B virus detection based on tin-doped WO3/In2O3 heterojunction nanowire photoelectrode under laser amplification. Anal Bioanal Chem, 2020, 412, 5367 doi: 10.1007/s00216-020-02752-z[66] Manzano M, Viezzi S, Mazerat S, et al. Rapid and label-free electrochemical DNA biosensor for detecting hepatitis A virus. Biosens Bioelectron, 2018, 100, 89 doi: 10.1016/j.bios.2017.08.043[67] Lim R R X, Bonanni A. The potential of electrochemistry for the detection of coronavirus-induced infections. Trac Trends Anal Chem, 2020, 133, 116081 doi: 10.1016/j.trac.2020.116081[68] Pumford E A, Lu J K, Spaczai I, et al. Developments in integrating nucleic acid isothermal amplification and detection systems for point-of-care diagnostics. Biosens Bioelectron, 2020, 170, 112674 doi: 10.1016/j.bios.2020.112674 -
Proportional views





 DownLoad:
DownLoad:










 Xiaofei Liu:got her Master's Degree on Veterinary in 2014 at Jilin University. Her research interests include detection and monitoring technologies for animal diseases, animal disease risk analysis and genetically modified animals detection
Xiaofei Liu:got her Master's Degree on Veterinary in 2014 at Jilin University. Her research interests include detection and monitoring technologies for animal diseases, animal disease risk analysis and genetically modified animals detection Shaoqiang Wu:got his Doctor's Degree on Veterinary Parasitology in 2003 at China Agricultural University. His research interests include precision detection technology for biosecurity risk, on-site detection techniques for shellfish parasites and aquatic animal diseases
Shaoqiang Wu:got his Doctor's Degree on Veterinary Parasitology in 2003 at China Agricultural University. His research interests include precision detection technology for biosecurity risk, on-site detection techniques for shellfish parasites and aquatic animal diseases Xiangmei Lin:got her Doctor's Degree on Veterinary Pathology in 1998 at Nanjing Agricultural University. Her research interests include detection and monitoring technologies for animal diseases, zoonotic diseases, foreign animal diseases and monitoring technologies of genetically modified animals detection
Xiangmei Lin:got her Doctor's Degree on Veterinary Pathology in 1998 at Nanjing Agricultural University. Her research interests include detection and monitoring technologies for animal diseases, zoonotic diseases, foreign animal diseases and monitoring technologies of genetically modified animals detection



