| Citation: |
Khushnuma Asghar, D. Das. Effect of polishing parameters on abrasive free chemical mechanical planarization of semi-polar(11$\bar{2}$2) aluminum nitride surface[J]. Journal of Semiconductors, 2016, 37(3): 036001. doi: 10.1088/1674-4926/37/3/036001
****
K Asghar, D. Das. Effect of polishing parameters on abrasive free chemical mechanical planarization of semi-polar(11$\bar{2}$2) aluminum nitride surface[J]. J. Semicond., 2016, 37(3): 036001. doi: 10.1088/1674-4926/37/3/036001.
|
Effect of polishing parameters on abrasive free chemical mechanical planarization of semi-polar(11$\bar{2}$2) aluminum nitride surface
DOI: 10.1088/1674-4926/37/3/036001
More Information
-
Abstract
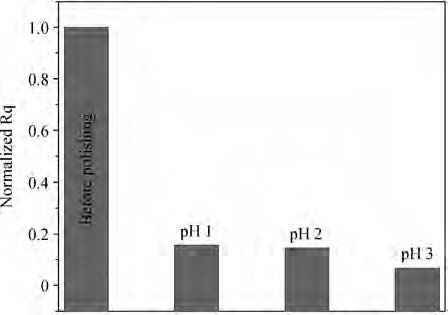
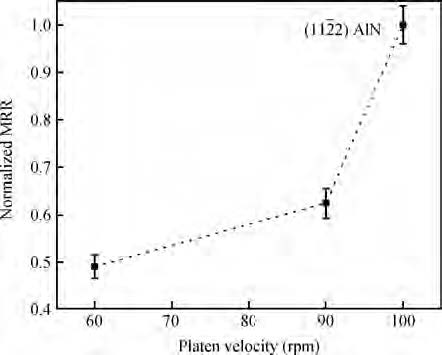
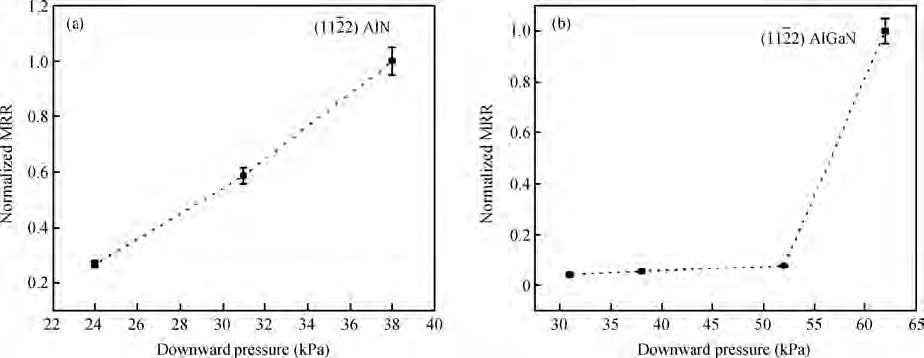
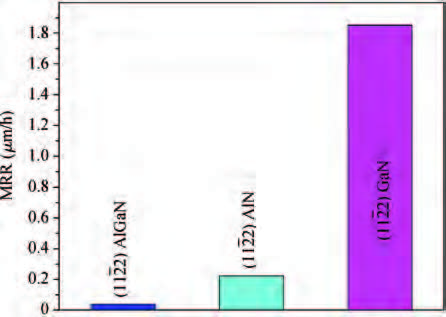
An abrasive free chemical mechanical planarization(AFCMP) of semi-polar(11$\bar{2}$2) AlN surface has been demonstrated. The effect of slurry pH, polishing pressure, and platen velocity on the material removal rate(MRR) and surface quality(RMS roughness) have been studied. The effect of polishing pressure on the AFCMP of the(11$\bar{2}$2) AlN surface has been compared with that of the(11$\bar{2}$2) AlGaN surface. The maximum MRR has been found to be ~562 nm/h for the semi-polar(11$\bar{2}$2) AlN surface, under the experimental conditions of 38 kPa pressure, 90 rpm platen velocity, 30 rpm carrier velocity, slurry pH 3 and 0.4 M oxidizer concentration. The best root mean square(RMS) surface roughness of ~1.2 nm and ~0.7 nm, over a large scanning area of 0.70×0.96 mm2, has been achieved on AFCMP processed semi-polar(11$\bar{2}$2) AlN and(AlGaN) surfaces using optimized slurry chemistry and processing parameters. -
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] -
Proportional views






 DownLoad:
DownLoad: