| Citation: |
Shengli Wang, Kangda Yin, Xiang Li, Hongwei Yue, Yunling Liu. Planarization mechanism of alkaline copper CMP slurry based on chemical mechanical kinetics[J]. Journal of Semiconductors, 2013, 34(8): 086003. doi: 10.1088/1674-4926/34/8/086003
****
S L Wang, K D Yin, X Li, H W Yue, Y L Liu. Planarization mechanism of alkaline copper CMP slurry based on chemical mechanical kinetics[J]. J. Semicond., 2013, 34(8): 086003. doi: 10.1088/1674-4926/34/8/086003.
|
Planarization mechanism of alkaline copper CMP slurry based on chemical mechanical kinetics
DOI: 10.1088/1674-4926/34/8/086003
More Information
-
Abstract
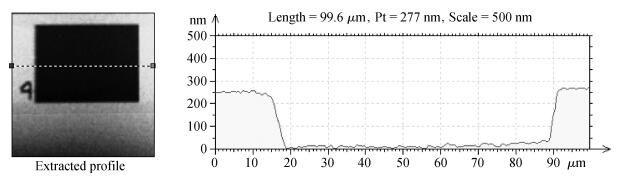
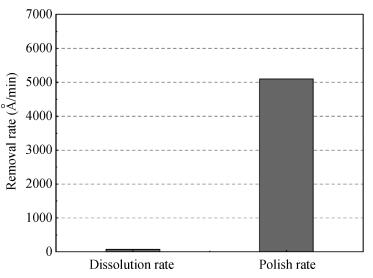
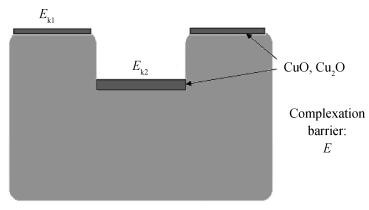
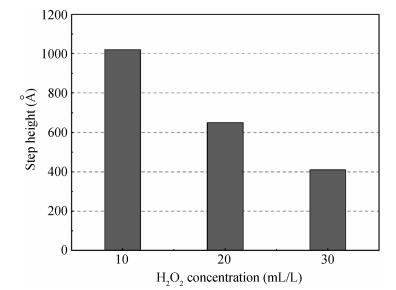
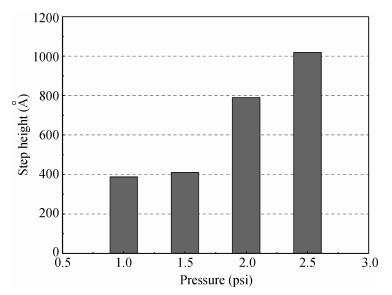
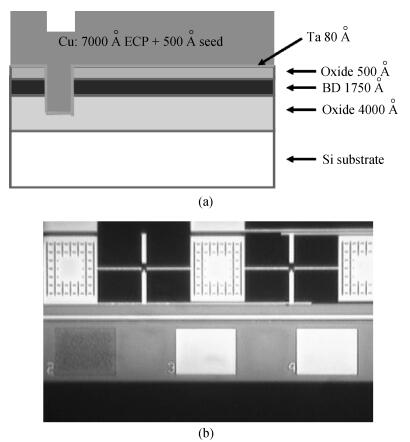
The planarization mechanism of alkaline copper slurry is studied in the chemical mechanical polishing (CMP) process from the perspective of chemical mechanical kinetics. Different from the international dominant acidic copper slurry, the copper slurry used in this research adopted the way of alkaline technology based on complexation. According to the passivation property of copper in alkaline conditions, the protection of copper film at the concave position on a copper pattern wafer surface can be achieved without the corrosion inhibitors such as benzotriazole (BTA), by which the problems caused by BTA can be avoided. Through the experiments and theories research, the chemical mechanical kinetics theory of copper removal in alkaline CMP conditions was proposed. Based on the chemical mechanical kinetics theory, the planarization mechanism of alkaline copper slurry was established. In alkaline CMP conditions, the complexation reaction between chelating agent and copper ions needs to break through the reaction barrier. The kinetic energy at the concave position should be lower than the complexation reaction barrier, which is the key to achieve planarization. -
References
[1] Hu Y, Liu Y L, Liu X Y, et al. Effect of alkaline slurry on the electric character of the pattern Cu wafer. Journal of Semiconductors, 2011, 32(7):076002 doi: 10.1088/1674-4926/32/7/076002[2] Ruan W B, Chen L, Li Z G, et al. Effects of pattern characteristics on copper CMP. Journal of Semiconductors, 2009, 30(4):046001 doi: 10.1088/1674-4926/30/4/046001[3] Li X, Liu Y L, Wang C W, et al. Study of the stability of H2O2 in alkaline slurry. Adv Semicond Manuf Technol, 2012, 37(11):850[4] Yin K D, Wang S L, Liu Y L, et al. Impact of the temperature and mass transfer on the removal rate uniformity in copper CMP process. Adv Semicond Manuf Technol, 2012, 37(10):768[5] Fayolle M, Romagna F. Copper CMP evaluation:planarization issues. Microelectron Eng, 1997, 37/38:135 doi: 10.1016/S0167-9317(97)00104-4[6] Pandija S, Roy D, Babu S V. Chemical mechanical planarization of copper using abrasive-free solutions of oxalic acid and hydro-gen peroxide. Mater Chem Phys, 2007, 102(2/3):144[7] Price D T, Gutmann R J, Murarka S P. Damascene copper interconnects with polymer ILDs. Thin Solid Films, 1997, 308/309:523[8] Kriz J, Angelkort C, Czekalla M, et al. Overview of dual damascene integration schemes in Cu BEOL integration. Microelectron Eng, 2008, 85(10):2128 doi: 10.1016/j.mee.2008.05.034[9] Su J X, Du J X, Ma L J, et al. Material removal rate of 6H-SiC crystal substrate CMP using an alumina (Al2O3) abrasive. Journal of Semiconductors, 2012, 33(10):106003 doi: 10.1088/1674-4926/33/10/106003[10] Wang C W, Liu Y L, Niu X H, et al. An advanced alkaline slurry for barrier chemical mechanical planarization on patterned wafers. Journal of Semiconductors, 2012, 33(4):046001 doi: 10.1088/1674-4926/33/4/046001[11] Wang C W, Liu Y L, Tian J Y, et al. Planarization properties of an alkaline slurry without an inhibitor on copper patterned wafer CMP. Journal of Semiconductors, 2012, 33(11):116001 doi: 10.1088/1674-4926/33/11/116001 -
Proportional views





 DownLoad:
DownLoad: