| Citation: |
M. Benhaliliba, A. Tiburcio-Silver, A. Avila-Garcia, A. Tavira, Y. S. Ocak, M. S. Aida, C. E. Benouis. The sprayed ZnO films: nanostructures and physical parameters[J]. Journal of Semiconductors, 2015, 36(8): 083001. doi: 10.1088/1674-4926/36/8/083001
****
M. Benhaliliba, A. Tiburcio-Silver, A. Avila-Garcia, A. Tavira, Y. S. Ocak, M. S. Aida, C. E. Benouis. The sprayed ZnO films: nanostructures and physical parameters[J]. J. Semicond., 2015, 36(8): 083001. doi: 10.1088/1674-4926/36/8/083001.
|
The sprayed ZnO films: nanostructures and physical parameters
DOI: 10.1088/1674-4926/36/8/083001
More Information
-
Abstract
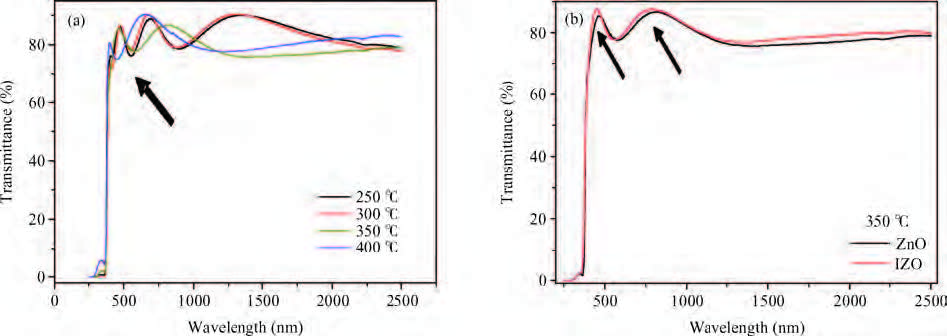
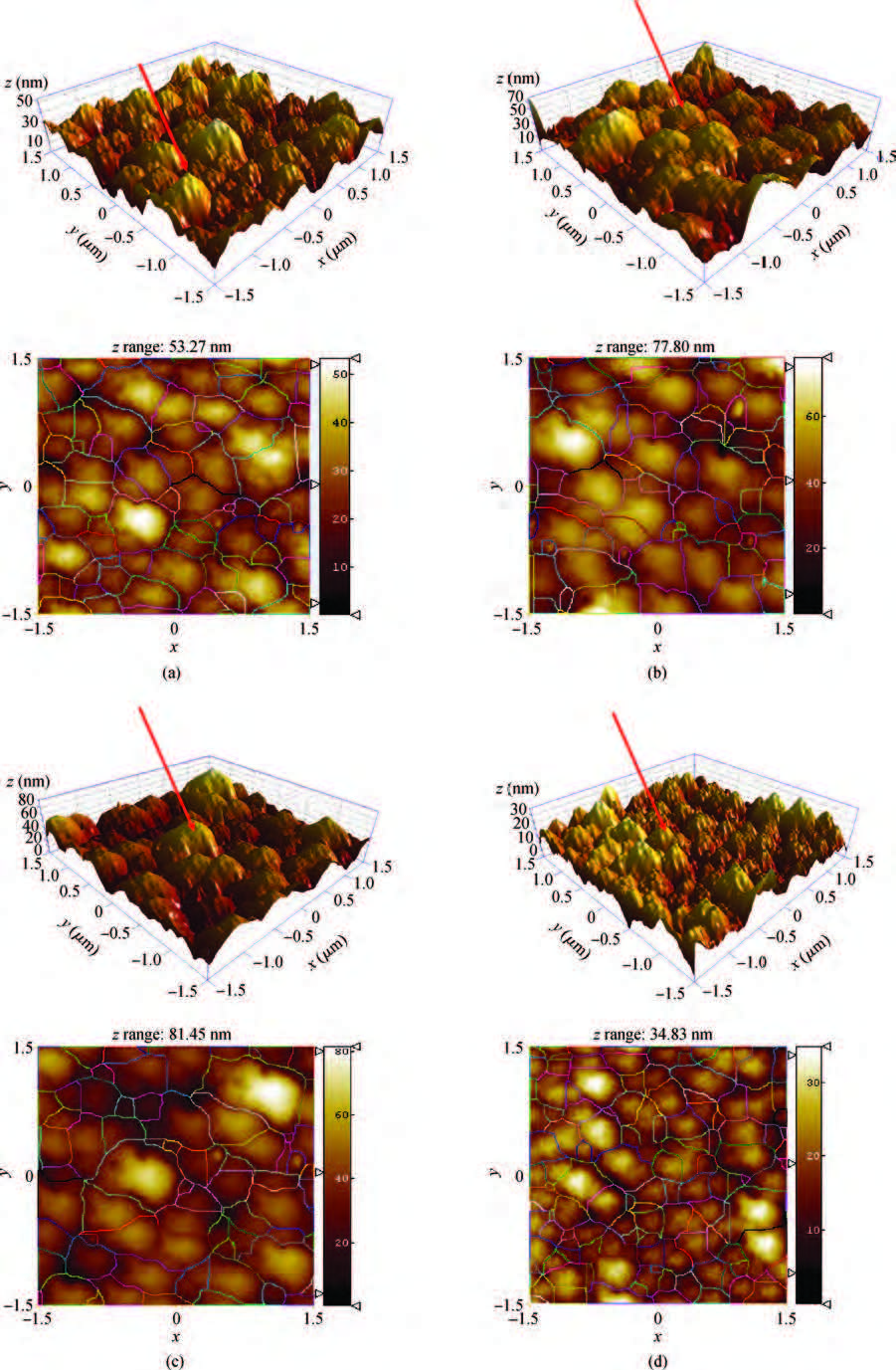
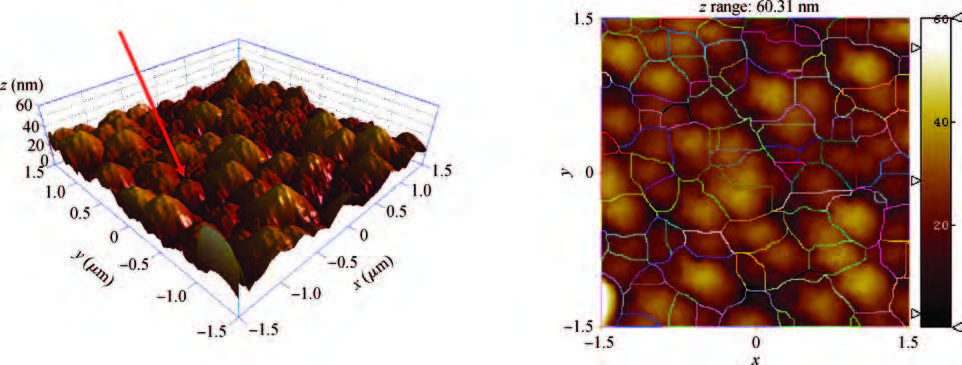
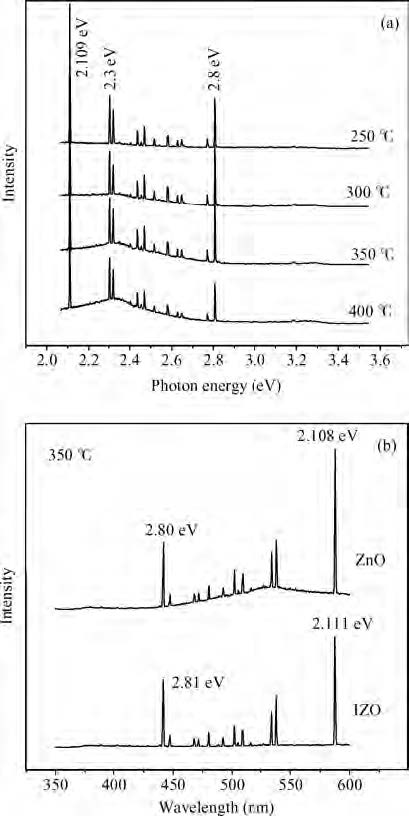
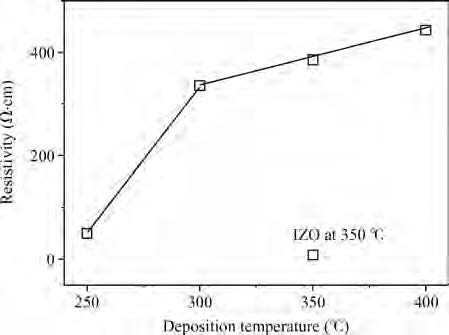
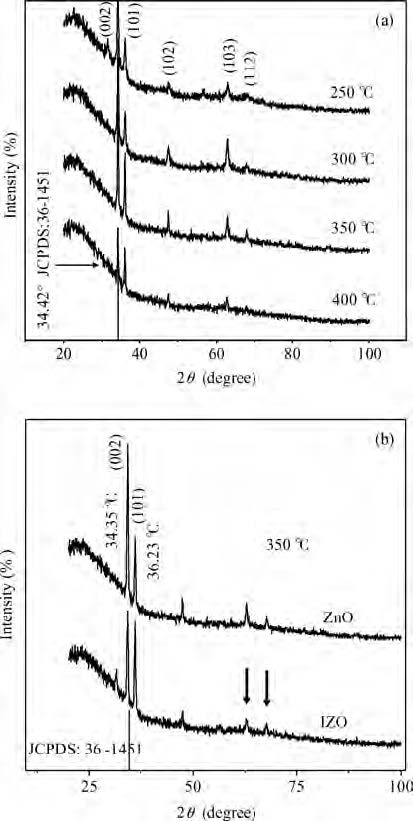
We synthesized the pure and indium-doped (IZO) ZnO films with a facile composition control spray pyrolysis route. The substrate temperature (Ts) and In-doping effects on the properties of as-grown films are investigated. The X-ray pattern confirms that the as-synthesized ZnO phase is grown along a (002) preferential plane. It is revealed that the crystalline structure is improved with a substrate temperature of 350 ℃. Moreover, the morphology of as-grown films, analyzed by AFM, shows nanostructures that have grown along the c-axis. The (3 × 3μm2) area scanned AFM surface studies give the smooth film surface RMS <40 nm. The UV-VIS-IR measurements reveal that the sprayed films are highly transparent in the visible and IR bands. The photoluminescence analysis shows that the strong blue and yellow luminescences of 2.11 and 2.81 eV are emitted from ZnO and IZO films with a slight shift in photon energy caused by In-doping. The band gap is a bit widened by In-doping, 3.21 eV (ZnO) and 3.31 eV (IZO) and the resistivity is reduced from 385 to 8 Ω ·m. An interesting result is the resistivity linear dependence on the substrate temperature of pure ZnO films. -
References
[1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] [21] [22] -
Proportional views





 DownLoad:
DownLoad: