| Citation: |
Yang Liu, Kunyuan Lu, Yujie Zhu, Xudong Hu, Yusheng Li, Guozheng Shi, Xingyu Zhou, Lin Yuan, Xiang Sun, Xiaobo Ding, Irfan Ullah Muhammad, Qing Shen, Zeke Liu, Wanli Ma. Colloidal synthesis of lead chalcogenide/lead chalcohalide core/shell nanostructures and structural evolution[J]. Journal of Semiconductors, 2025, 46(4): 042101. doi: 10.1088/1674-4926/24050026
****
Y Liu, K Y Lu, Y J Zhu, X D Hu, Y S Li, G Z Shi, X Y Zhou, L Yuan, X Sun, X B Ding, I U Muhammad, Q Shen, Z K Liu, and W L Ma, Colloidal synthesis of lead chalcogenide/lead chalcohalide core/shell nanostructures and structural evolution[J]. J. Semicond., 2025, 46(4), 042101 doi: 10.1088/1674-4926/24050026
|
Colloidal synthesis of lead chalcogenide/lead chalcohalide core/shell nanostructures and structural evolution
DOI: 10.1088/1674-4926/24050026
CSTR: 32376.14.1674-4926.24050026
More Information-
Abstract
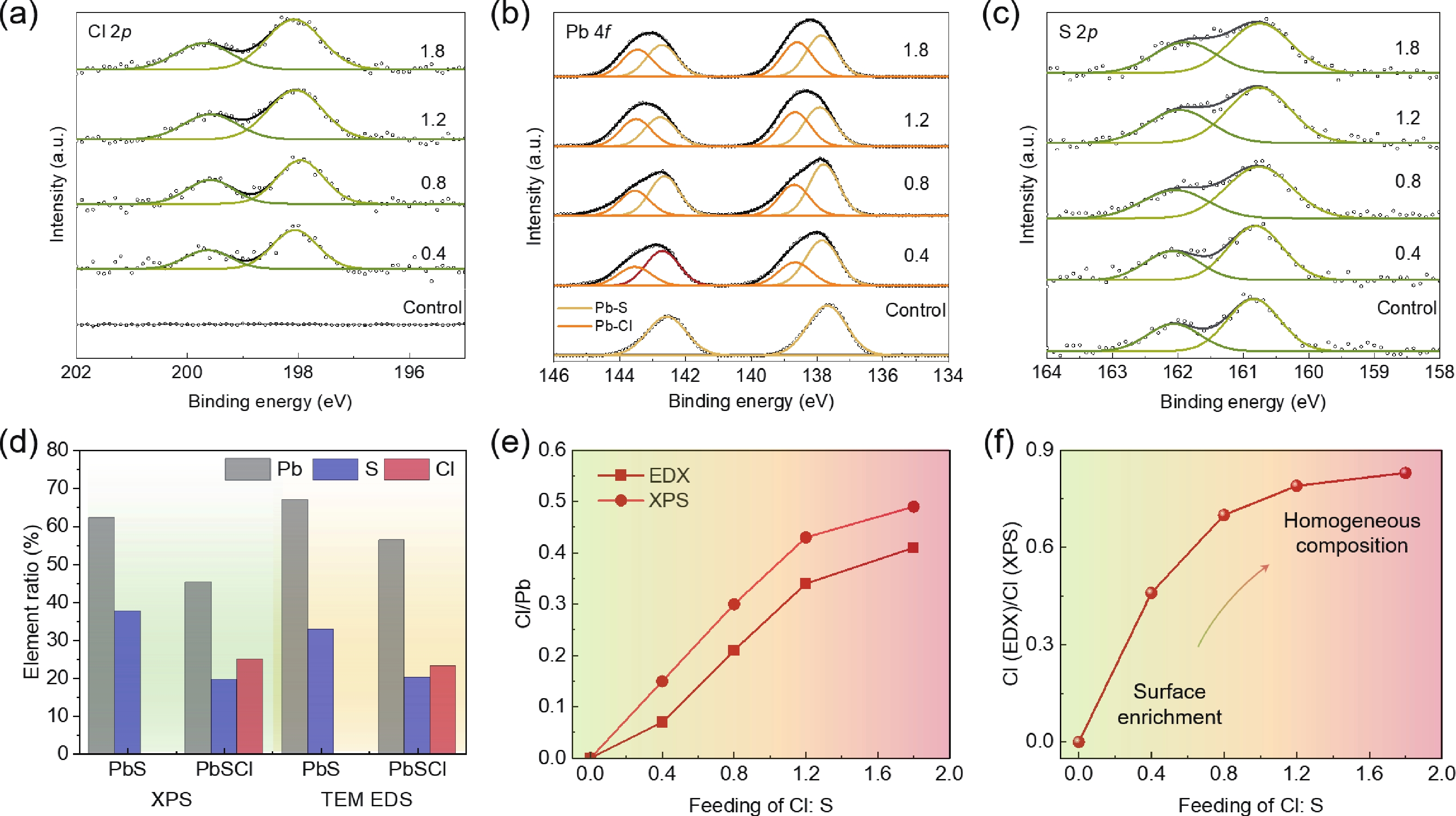
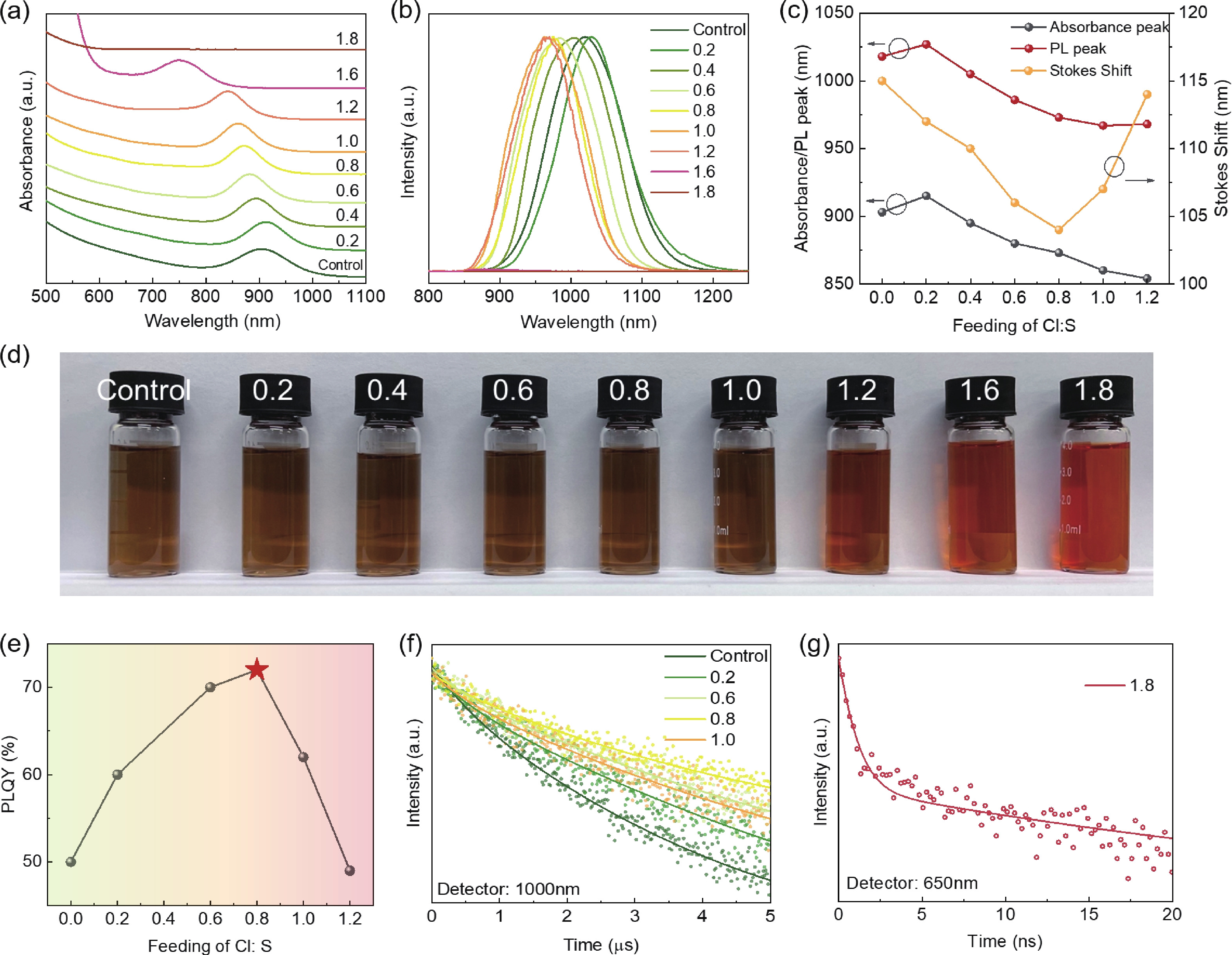
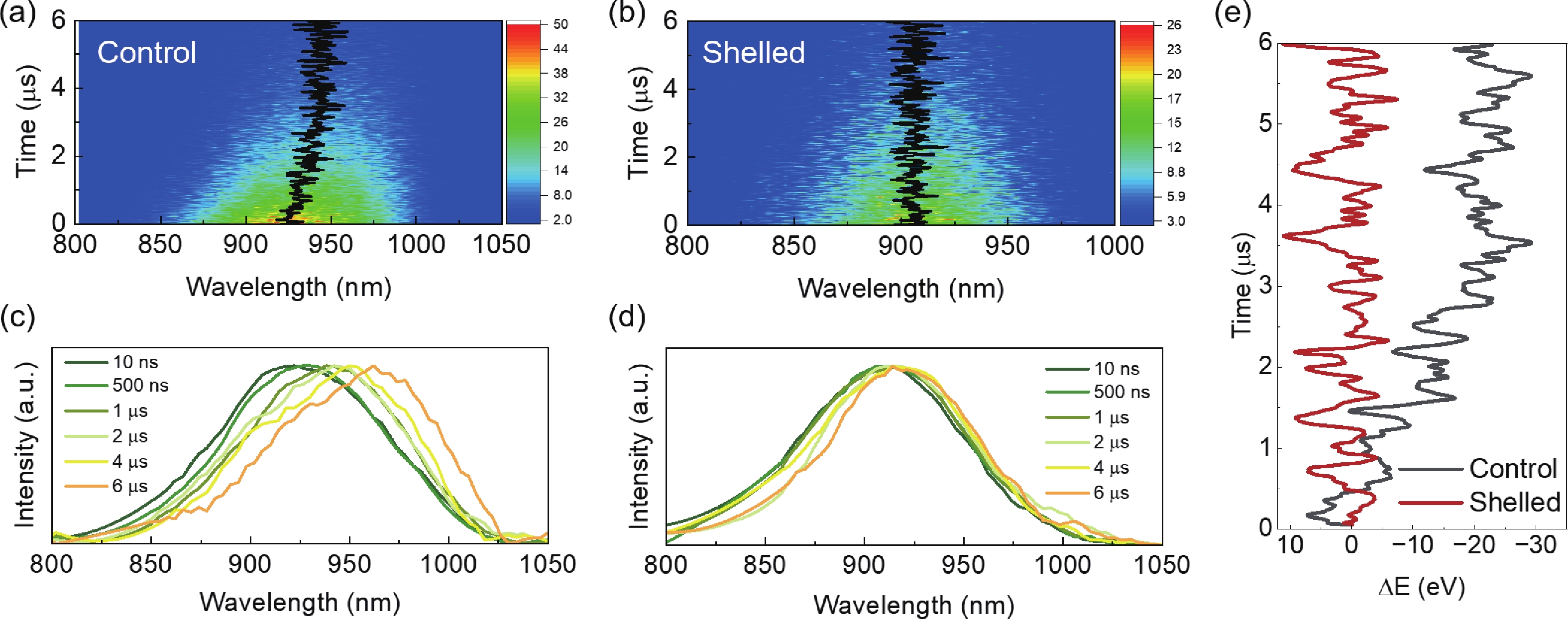
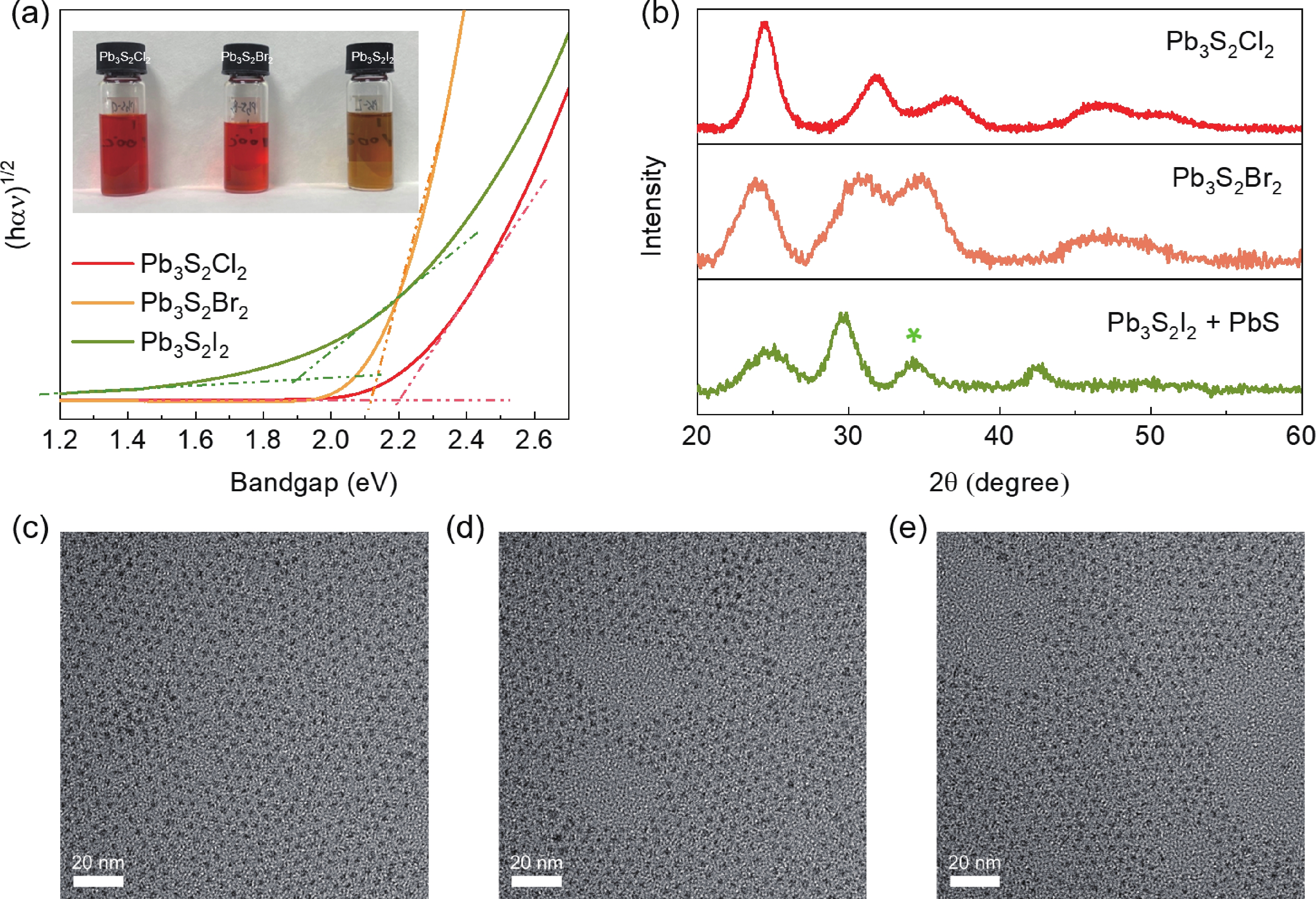
Lead chalcohalides (PbYX, X = Cl, Br, I; Y = S, Se) is an extension of the classic Pb chalcogenides (PbY). Constructing the heterogeneous integration with PbYX and PbY material systems makes it possible to achieve significantly improved optoelectronic performance. In this work, we studied the effect of introducing halogen precursors on the structure of classical PbS nanocrystals (NCs) during the synthesis process and realized the preparation of PbS/Pb3S2X2 core/shell structure for the first time. The core/shell structure can effectively improve their optical properties. Furthermore, our approach enables the synthesis of Pb3S2Br2 that had not yet been reported. Our results not only provide valuable insights into the heterogeneous integration of PbYX and PbY materials to elevate material properties but also provide an effective method for further expanding the preparation of PbYX material systems. -
References
[1] Ghorpade U V, Suryawanshi M P, Green M A, et al. Emerging chalcohalide materials for energy applications. Chem Rev, 2023, 123, 327 doi: 10.1021/acs.chemrev.2c00422[2] Wlaźlak E, Blachecki A, Bisztyga-Szklarz M, et al. Heavy pnictogen chalcohalides: The synthesis, structure and properties of these rediscovered semiconductors. Chem Commun, 2018, 54, 12133 doi: 10.1039/C8CC05149F[3] Mihailovic D. Inorganic molecular wires: Physical and functional properties of transition metal chalco-halide polymers. Prog Mater Sci, 2009, 54, 309 doi: 10.1016/j.pmatsci.2008.09.001[4] Xu B, Feng T L, Agne M T, et al. Manipulating band structure through reconstruction of binary metal sulfide for high-performance thermoelectrics in solution-synthesized nanostructured Bi13S18I2. Angew Chem Int Ed, 2018, 57, 2413 doi: 10.1002/anie.201713223[5] Quarta D, Toso S, Giannuzzi R, et al. Colloidal bismuth chalcohalide nanocrystals. Angew Chem Int Ed, 2022, 61, e202201747 doi: 10.1002/anie.202201747[6] Johnsen S, Liu Z F, Peters J A, et al. Thallium chalcohalides for X-ray and γ-ray detection. J Am Chem Soc, 2011, 133, 10030 doi: 10.1021/ja202540t[7] Nie R M, Im J, Seok S I. Efficient solar cells employing light-harvesting Sb0.67Bi0.33SI. Adv Mater, 2019, 31, 1808344 doi: 10.1002/adma.201808344[8] Nie R M, Lee K S, Hu M M, et al. Heteroleptic tin-antimony sulfoiodide for stable and lead-free solar cells. Matter, 2020, 3, 1701 doi: 10.1016/j.matt.2020.08.020[9] Nie R M, Kim B, Hong S T, et al. Nanostructured heterojunction solar cells based on Pb2SbS2I3: Linking lead halide perovskites and metal chalcogenides. ACS Energy Lett, 2018, 3, 2376 doi: 10.1021/acsenergylett.8b01332[10] Akkerman Q A, Rainò G, Kovalenko M V, et al. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals. Nat Mater, 2018, 17, 394 doi: 10.1038/s41563-018-0018-4[11] Liu Y, Shi G Z, Liu Z K, et al. Toward printable solar cells based on PbX colloidal quantum dot inks. Nanoscale Horiz, 2021, 6, 8 doi: 10.1039/D0NH00488J[12] Carey G H, Abdelhady A L, Ning Z J, et al. Colloidal quantum dot solar cells. Chem Rev, 2015, 115, 12732 doi: 10.1021/acs.chemrev.5b00063[13] Saran R, Curry R J. Lead sulphide nanocrystal photodetector technologies. Nat Photonics, 2016, 10, 81 doi: 10.1038/nphoton.2015.280[14] Voznyy O, Sutherland B R, Ip A H, et al. Engineering charge transport by heterostructuring solution-processed semiconductors. Nat Rev Mater, 2017, 2, 17026 doi: 10.1038/natrevmats.2017.26[15] Li D P, Ma J R, Liu W B, et al. Enhancing performance of inverted quantum-dot light-emitting diodes based on a solution-processed hole transport layer via ligand treatment. J Semicond, 2023, 44, 092603 doi: 10.1088/1674-4926/44/9/092603[16] Qu X W, Sun X W. Impedance spectroscopy for quantum dot light-emitting diodes. J Semicond, 2023, 44, 091603 doi: 10.1088/1674-4926/44/9/091603[17] Fenner J, Rabenau A, Trageser G. Solid-state chemistry of thio-, seleno-, and tellurohalides of representative and transition elements. Advances in Inorganic Chemistry and Radiochemistry. Amsterdam: Elsevier, 1980, 329[18] Krebs B. Die kristallstrukturen von Pb4SeBr6, Pb5S2J6 and Pb7S2Br10. Z Für Anorg Und Allg Chem, 1973, 396, 137[19] Toso S, Akkerman Q A, Martín-García B, et al. Nanocrystals of lead chalcohalides: A series of kinetically trapped metastable nanostructures. J Am Chem Soc, 2020, 142, 10198 doi: 10.1021/jacs.0c03577[20] Imran M, Peng L C, Pianetti A, et al. Halide perovskite–lead chalcohalide nanocrystal heterostructures. J Am Chem Soc, 2021, 143, 1435 doi: 10.1021/jacs.0c10916[21] Dutta S K, Peng L C, Hudait B, et al. Halide perovskite cluster precursors: A paradigm for obtaining structure- and color-tunable light-emitting nanocrystals. ACS Energy Lett, 2022, 7, 3177 doi: 10.1021/acsenergylett.2c01638[22] Qiu H W, Li F, He S, et al. Epitaxial CsPbBr3/CdS Janus nanocrystal heterostructures for efficient charge separation. Adv Sci, 2023, 10, 2206560 doi: 10.1002/advs.202206560[23] Toso S, Imran M, Mugnaioli E, et al. Halide perovskites as disposable epitaxial templates for the phase-selective synthesis of lead sulfochloride nanocrystals. Nat Commun, 2022, 13, 3976 doi: 10.1038/s41467-022-31699-1[24] Vafaie M, Fan J Z, Morteza Najarian A, et al. Colloidal quantum dot photodetectors with 10-ns response time and 80% quantum efficiency at 1, 550nm. Matter, 2021, 4, 1042 doi: 10.1016/j.matt.2020.12.017[25] Liu M X, Voznyy O, Sabatini R, et al. Hybrid organic-inorganic inks flatten the energy landscape in colloidal quantum dot solids. Nat Mater, 2017, 16, 258 doi: 10.1038/nmat4800[26] Shen W S, Liu Y, Grater L, et al. Thickness-variation-insensitive near-infrared quantum dot LEDs. Sci Bull, 2023, 68, 2954 doi: 10.1016/j.scib.2023.10.018[27] Pradhan S, Dalmases M, Taghipour N, et al. Colloidal quantum dot light emitting diodes at telecom wavelength with 18% quantum efficiency and over 1 MHz bandwidth. Adv Sci, 2022, 9, 2200637 doi: 10.1002/advs.202200637[28] Liu Y, Gao Y Y, Yang Q, et al. Breaking the size limitation of directly-synthesized PbS quantum dot inks toward efficient short-wavelength infrared optoelectronic applications. Angew Chem Int Ed, 2023, 62, e202300396 doi: 10.1002/anie.202300396[29] Woo J Y, Ko J H, Song J H, et al. Ultrastable PbSe nanocrystal quantum dots via in situ formation of atomically thin halide adlayers on PbSe(100). J Am Chem Soc, 2014, 136, 8883 doi: 10.1021/ja503957r[30] Moreels I, Justo Y, De Geyter B, et al. Size-tunable, bright, and stable PbS quantum dots: A surface chemistry study. ACS Nano, 2011, 5, 2004 doi: 10.1021/nn103050w[31] Green P B, Li Z Q, Wilson M W B. PbS nanocrystals made with excess PbCl2 have an intrinsic shell that reduces their stokes shift. J Phys Chem Lett, 2019, 10, 5897 doi: 10.1021/acs.jpclett.9b01841[32] Green P B, Villanueva F Y, Demmans K Z, et al. PbS nanocrystals made using excess lead chloride have a halide-perovskite-like surface. Chem Mater, 2021, 33, 9270 doi: 10.1021/acs.chemmater.1c02962[33] Brittman S, Colbert A E, Brintlinger T H, et al. Effects of a lead chloride shell on lead sulfide quantum dots. J Phys Chem Lett, 2019, 10, 1914 doi: 10.1021/acs.jpclett.9b00786[34] Hines M A, Scholes G D. Colloidal PbS nanocrystals with size-tunable near-infrared emission: Observation of post-synthesis self-narrowing of the particle size distribution. Adv Mater, 2003, 15, 1844 doi: 10.1002/adma.200305395[35] Bae W K, Char K, Hur H, et al. Single-step synthesis of quantum dots with chemical composition gradients. Chem Mater, 2008, 20, 531 doi: 10.1021/cm070754d[36] Nan W N, Niu Y, Qin H Y, et al. Crystal structure control of zinc-blende CdSe/CdS core/shell nanocrystals: Synthesis and structure-dependent optical properties. J Am Chem Soc, 2012, 134, 19685 doi: 10.1021/ja306651x[37] Lechner R T, Fritz-Popovski G, Yarema M, et al. Crystal phase transitions in the shell of PbS/CdS core/shell nanocrystals influences photoluminescence intensity. Chem Mater, 2014, 26, 5914 doi: 10.1021/cm502521q[38] Liu Z K, Zhong Y X, Shafei I, et al. Tuning infrared plasmon resonances in doped metal-oxide nanocrystals through cation-exchange reactions. Nat Commun, 2019, 10, 1394 doi: 10.1038/s41467-019-09165-2[39] Sagar L K, Walravens W, Zhao Q, et al. PbS/CdS core/shell quantum dots by additive, layer-by-layer shell growth. Chem Mater, 2016, 28, 6953 doi: 10.1021/acs.chemmater.6b02638[40] Fisher B, Caruge J M, Zehnder D, et al. Room-temperature ordered photon emission from multiexciton states in single CdSe core-shell nanocrystals. Phys Rev Lett, 2005, 94, 087403 doi: 10.1103/PhysRevLett.94.087403[41] Jin L, Sirigu G, Tong X, et al. Engineering interfacial structure in "Giant" PbS/CdS quantum dots for photoelectrochemical solar energy conversion. Nano Energy, 2016, 30, 531 doi: 10.1016/j.nanoen.2016.10.029[42] Imperiale C J, Yarur Villanueva F, Nikbin E, et al. Direct synthesis of ultrasmall PbS nanocrystals passivated with a metal-halide-perovskite-like monolayer. Chem Mater, 2024, 36, 4121 doi: 10.1021/acs.chemmater.3c01814[43] Ni D R, Guo S, Powderly K M, et al. A high-pressure phase with a non-centrosymmetric crystal structure in the PbSe–PbBr2 system. J Solid State Chem, 2019, 280, 120982 doi: 10.1016/j.jssc.2019.120982[44] Rabenau A, Rau H. Über sulfidhalogenide des bleis und das Pb4SeBr6. Z Für Anorg Und Allg Chem, 1969, 369, 295 -
Proportional views





 Yang Liu is currently a Ph.D. candidate under the supervision of Prof. Wanli Ma at Soochow University. He obtained his Master’s degree from Soochow University under the supervision of Prof. Wanli Ma in 2021. His current research interest focuses on the high-performance PbX quantum dot synthesis and optoelectronics applications.
Yang Liu is currently a Ph.D. candidate under the supervision of Prof. Wanli Ma at Soochow University. He obtained his Master’s degree from Soochow University under the supervision of Prof. Wanli Ma in 2021. His current research interest focuses on the high-performance PbX quantum dot synthesis and optoelectronics applications. Kunyuan Lu got her PhD from Soochow University in 2017. Now she is a postdoctor at Soochow University under the supervision of Prof. Wanli Ma. Her research focuses on PbS quantum dot solar cells.
Kunyuan Lu got her PhD from Soochow University in 2017. Now she is a postdoctor at Soochow University under the supervision of Prof. Wanli Ma. Her research focuses on PbS quantum dot solar cells. Zeke Liu is a professor at Soochow University. He obtained his PhD degree from Soochow University, worked as a joint PhD student under the supervision of Prof. Paul Alivisatos at University of California, Berkeley. Before he joined Soochow University in 2019, he worked as a joint postdoctoral scholar in Indiana University, Bloomington and Soochow University. His current research interest focuses on the design and synthesis of semiconductor quantum dots/nanocrystals, and their application in optoelectronic devices.
Zeke Liu is a professor at Soochow University. He obtained his PhD degree from Soochow University, worked as a joint PhD student under the supervision of Prof. Paul Alivisatos at University of California, Berkeley. Before he joined Soochow University in 2019, he worked as a joint postdoctoral scholar in Indiana University, Bloomington and Soochow University. His current research interest focuses on the design and synthesis of semiconductor quantum dots/nanocrystals, and their application in optoelectronic devices. Wanli Ma is currently a professor in the Institute of Functional Nano & Soft Materials (FUNSOM) at Soochow University. He received his PhD degree in 2006 from the University of California at Santa Barbara under the supervision of Prof. Alan J. Heeger. Before he joined Soochow University in 2010, he worked as a postdoctoral scholar in Prof. Paul Alivisatos’ group at Lawrence Berkeley national laboratory. His publications have been cited over 27 000 times. His current research interest focuses on developing solution processed solar cells, including quantum dots, organic materials and perovskite.
Wanli Ma is currently a professor in the Institute of Functional Nano & Soft Materials (FUNSOM) at Soochow University. He received his PhD degree in 2006 from the University of California at Santa Barbara under the supervision of Prof. Alan J. Heeger. Before he joined Soochow University in 2010, he worked as a postdoctoral scholar in Prof. Paul Alivisatos’ group at Lawrence Berkeley national laboratory. His publications have been cited over 27 000 times. His current research interest focuses on developing solution processed solar cells, including quantum dots, organic materials and perovskite.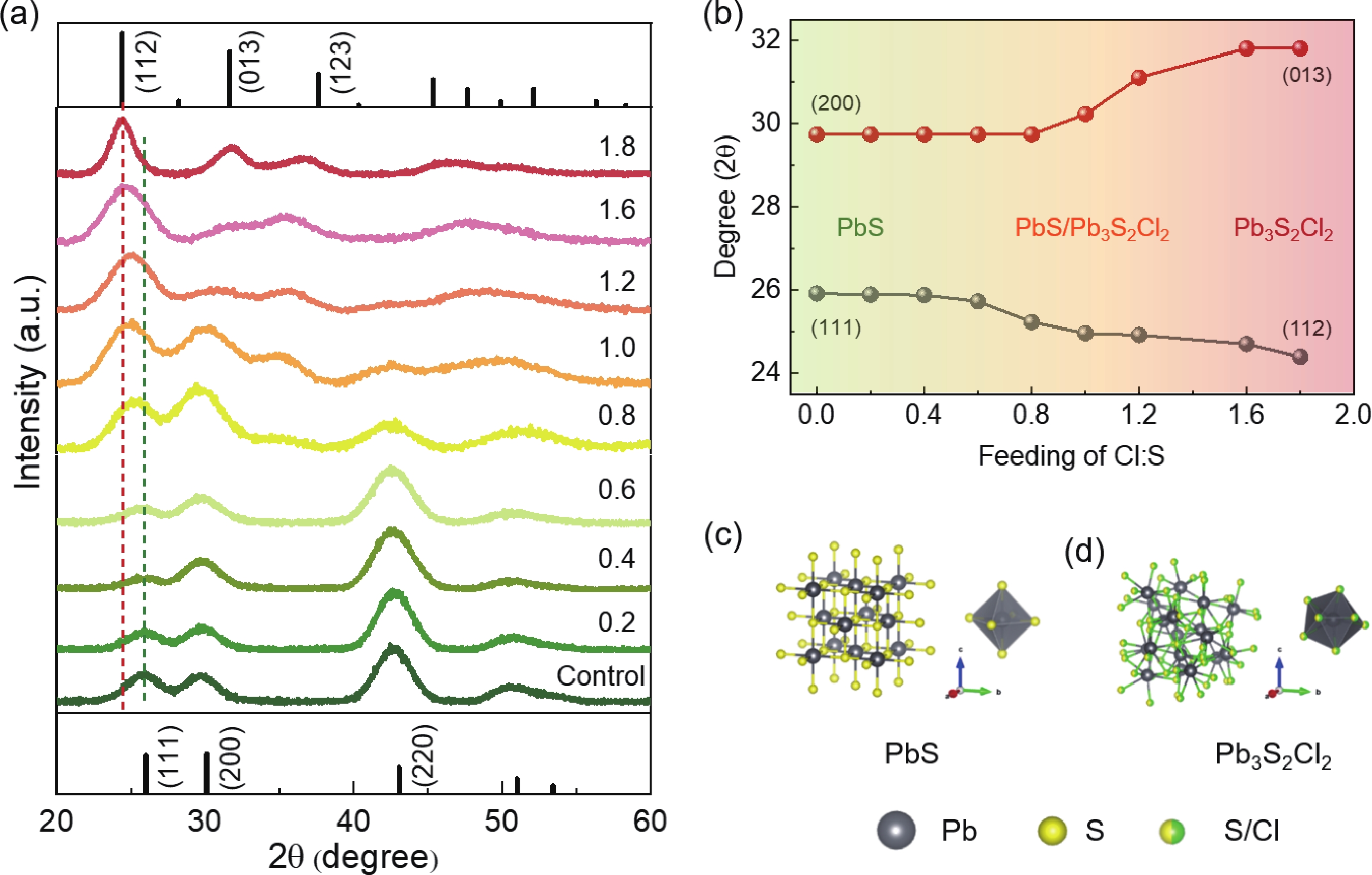
 DownLoad:
DownLoad: