| Citation: |
Aochen Wang, Shengli Wang, Yuling Liu, Yan Li. Electrochemical investigation of copper chemical mechanical planarization in alkaline slurry without an inhibitor[J]. Journal of Semiconductors, 2014, 35(2): 026003. doi: 10.1088/1674-4926/35/2/026003
****
A C Wang, S L Wang, Y L Liu, Y Li. Electrochemical investigation of copper chemical mechanical planarization in alkaline slurry without an inhibitor[J]. J. Semicond., 2014, 35(2): 026003. doi: 10.1088/1674-4926/35/2/026003.
|
Electrochemical investigation of copper chemical mechanical planarization in alkaline slurry without an inhibitor
DOI: 10.1088/1674-4926/35/2/026003
More Information
-
Abstract
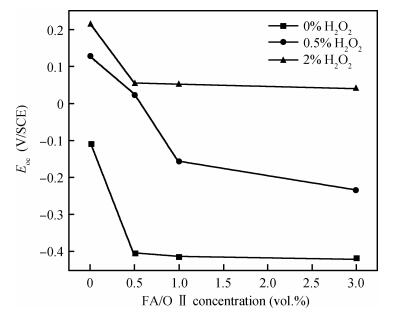
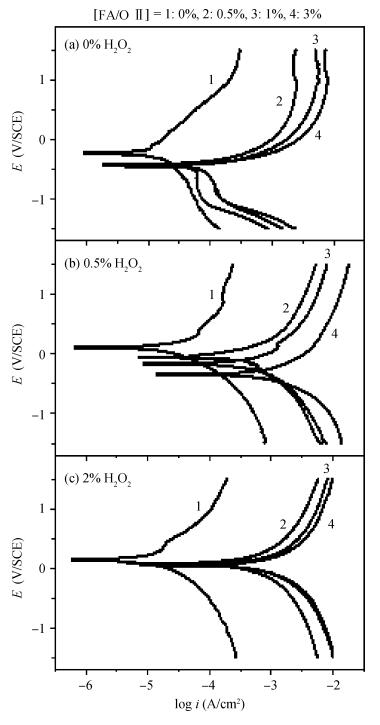
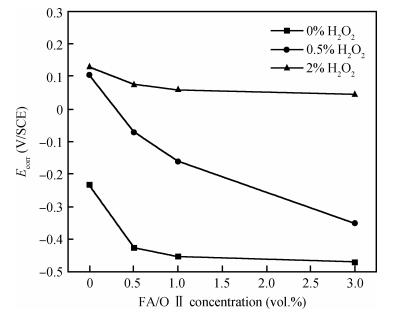
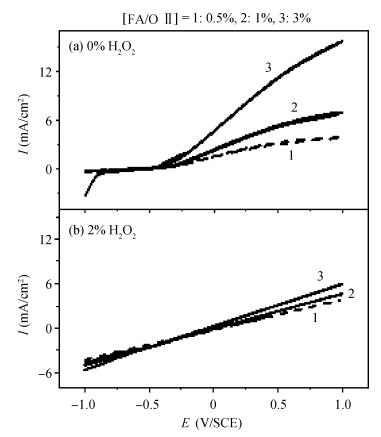
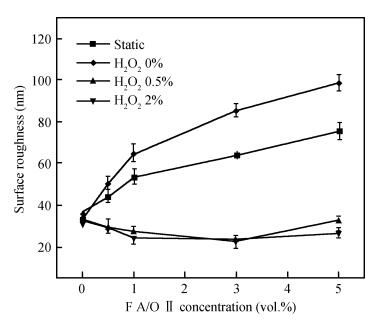
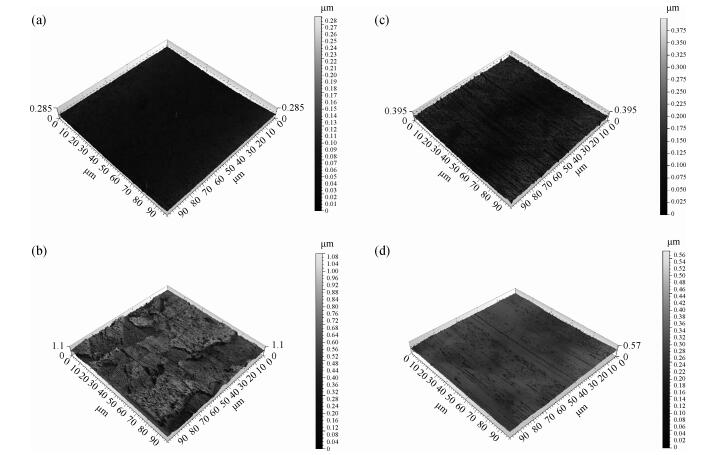
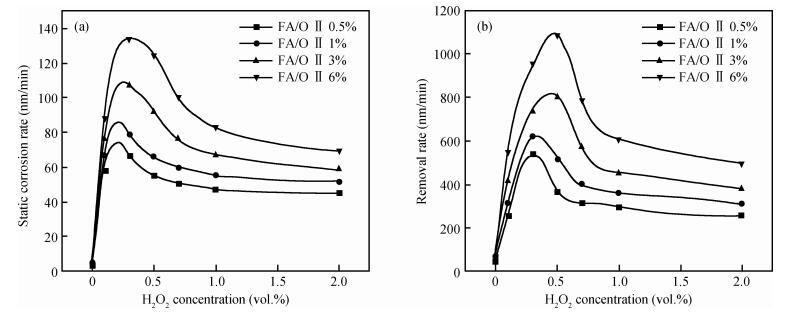
This work investigates the static corrosion and removal rates of copper as functions of H2O2 and FA/OⅡconcentration, and uses DC electrochemical measurements such as open circuit potential (OCP), Tafel analysis, as well as cyclic voltammetry (CV) to study H2O2 and FA/OⅡdependent surface reactions of Cu coupon electrode in alkaline slurry without an inhibitor. An atomic force microscopy (AFM) technique is also used to measure the surface roughness and surface morphology of copper in static corrosion and polishing conditions. It is shown that 0.5 vol.% H2O2 should be the primary choice to achieve high material removal rate. The electrochemical results reveal that the addition of FA/O Ⅱ can dissolve partial oxide film to accelerate the electrochemical anodic reactions and make the oxide layer porous, so that the structurally weak oxide film can be easily removed by mechanical abrasion. The variation of surface roughness and morphology of copper under static conditions is consistent with and provides further support for the reaction mechanisms proposed in the context of DC electrochemical measurements. In addition, in the presence of H2O2, 3 vol.% FA/O Ⅱ may be significantly effective from a surface roughness perspective to obtain a relatively flat copper surface in chemical mechanical planarization (CMP) process.-
Keywords:
- alkaline slurry,
- electrochemical,
- copper,
- CMP,
- surface roughness
-
References
[1] Zeidler D, Stavreva Z, Plotner M, et al. Characterization of Cu chemical mechanical polishing by electrochemical investigations. Microelectron Eng, 1997, 33:259 doi: 10.1016/S0167-9317(96)00053-6[2] Zantye P B, Kumar A, Sikdar A K. Chemical mechanical planarization for microelectronics applications. Mater Sci Eng R:Reports, 2004, 45:89 doi: 10.1016/j.mser.2004.06.002[3] Chen Y H, Tsai T H, Yen S C. Acetic acid and phosphoric acid adding to improve tantalum chemical mechanical polishing in hydrogen peroxide-based slurry. Microelectron Eng, 2010, 87(2):174 doi: 10.1016/j.mee.2009.07.009[4] Janjam S V S B, Peethala B C, Zheng J P, et al. Electrochemical investigation of surface reactions for chemically promoted chemical mechanical polishing of TaN in tartaric acid solutions. Mater Chem Phys, 2010, 123:521 doi: 10.1016/j.matchemphys.2010.05.008[5] Christopher M S, Dipankar R. Electrochemical characterization of surface complexes formed on Cu and Ta in succinic acid based solutions used for chemical mechanical planarization. Appl Surf Sci, 2010, 256(8):2583 doi: 10.1016/j.apsusc.2009.10.108[6] Kang M C, Kim Y J, Koo H C, et al. Local corrosion of the oxide passivation layer during Cu chemical mechanical polishing. Electrochem Solid-State Lett, 2009, 12(12):433 doi: 10.1149/1.3236391[7] Zheng J P, Roy D. Electrochemical examination of surface films formed during chemical mechanical planarization of copper in acetic and dodecyl sulfate solutions. Thin Solid Films, 2009, 517(16):4587 doi: 10.1016/j.tsf.2009.03.063[8] Nagendra P Y, Ramanathan S. Chemical mechanical planarization of copper in alkaline slurry with uric acid as inhibitor. Electrochimica Acta, 2007, 52(22):6353 doi: 10.1016/j.electacta.2007.04.044[9] Wang Chenwei, Liu Yuling, Tian Jianying, et al. Planarization properties of an alkaline slurry without an inhibitor on copper patterned wafer CMP. Journal of Semiconductors, 2012, 33(11):116001 doi: 10.1088/1674-4926/33/11/116001[10] Pourbaix M. Atlas of electrochemical equilibria in aqueous solutions. Huston:Natl Assn of Corrosion, 1975[11] Du T, Vijayakumar A, Desai V. Effect of hydrogen peroxide on oxidation of copper in CMP slurries containing glycine and Cu ions. Electrochimica Acta, 2004, 49(25):4505 doi: 10.1016/j.electacta.2004.05.008[12] Tsai T H, Yen S C. Localized corrosion effects and modifications of acidic and alkaline slurries on copper chemical mechanical polishing. Appl Surf Sci, 2003, 210(3/4):190 -
Proportional views





 DownLoad:
DownLoad: