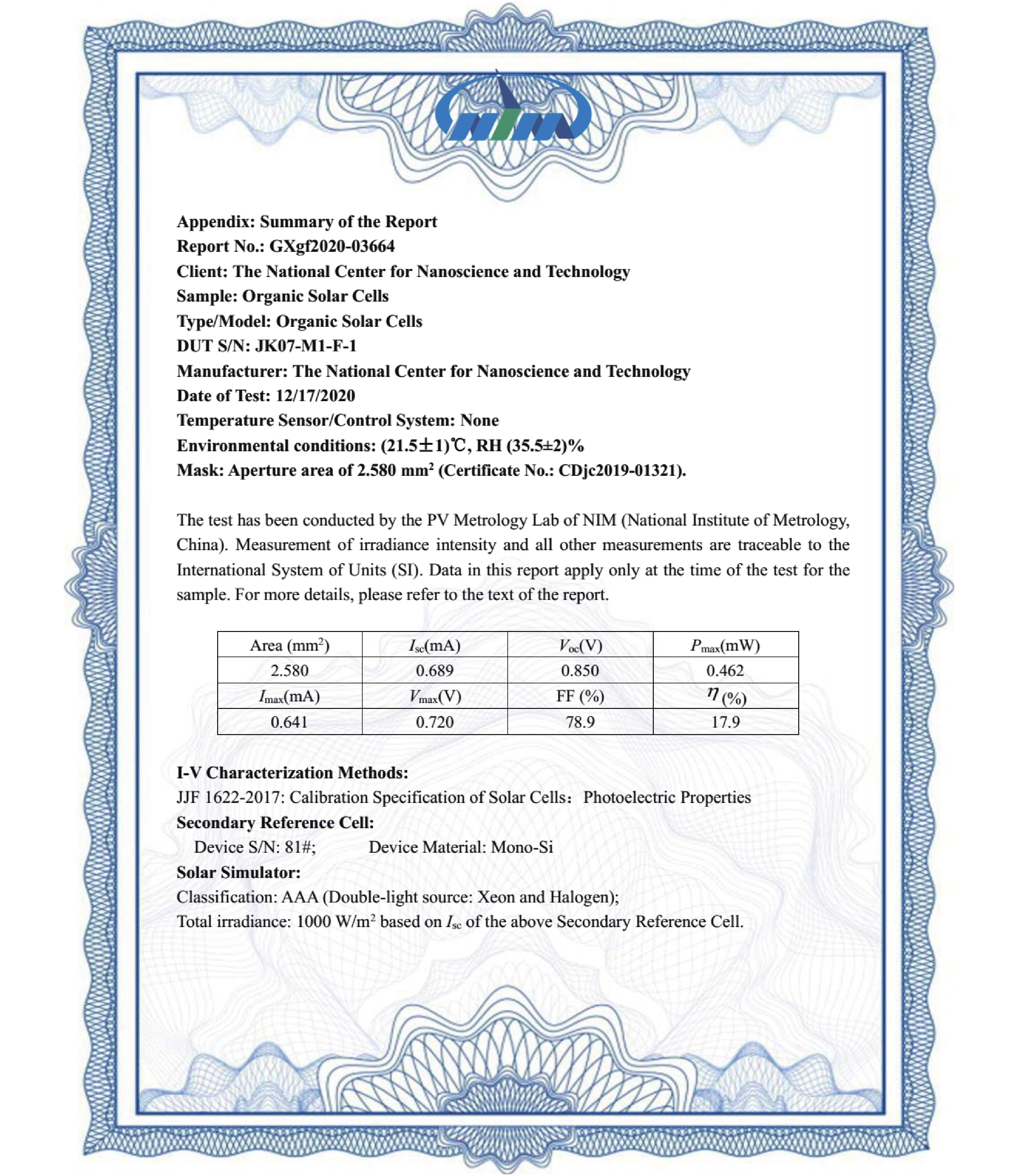
| Citation: |
Ke Jin, Zuo Xiao, Liming Ding. D18, an eximious solar polymer![J]. Journal of Semiconductors, 2021, 42(1): 010502. doi: 10.1088/1674-4926/42/1/010502
****
K Jin, Z Xiao, L M Ding, D18, an eximious solar polymer![J]. J. Semicond., 2021, 42(1): 010502. doi: 10.1088/1674-4926/42/1/010502.
|
-
References
[1] Tong Y, Xiao Z, Du X, et al. Progress of the key materials for organic solar cells. Sci China Chem, 2020, 63, 758 doi: 10.1007/s11426-020-9726-0[2] Tong Y, Xiao Z, Du X, et al. Progress of the key materials for organic solar cells. Sci Sin Chim, 2020, 50, 437 doi: 10.1360/SSC-2020-0018[3] Duan C, Ding L. The new era for organic solar cells: non-fullerene small molecular acceptors. Sci Bull, 2020, 65, 1231 doi: 10.1016/j.scib.2020.04.030[4] Duan C, Ding L. The new era for organic solar cells: polymer donors. Sci Bull, 2020, 65, 1422 doi: 10.1016/j.scib.2020.04.044[5] Duan C, Ding L. The new era for organic solar cells: polymer acceptors. Sci Bull, 2020, 65, 1508 doi: 10.1016/j.scib.2020.05.023[6] Duan C, Ding L. The new era for organic solar cells: small molecular donors. Sci Bull, 2020, 65, 1597 doi: 10.1016/j.scib.2020.05.019[7] Zhang Y, Duan C, Ding L. Indoor organic photovoltaics. Sci Bull, 2020, 65, 2040 doi: 10.1016/j.scib.2020.08.030[8] Wu J, Cheng S, Cheng Y, et al. Donor-acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem Soc Rev, 2015, 44, 1113 doi: 10.1039/C4CS00250D[9] Müllen K, Pisula W. Donor-acceptor polymers. J Am Chem Soc, 2015, 137, 9503 doi: 10.1021/jacs.5b07015[10] Zhou H, Yang L, You W. Rational design of high performance conjugated polymers for organic solar cells. Macromolecules, 2012, 45, 607 doi: 10.1021/ma201648t[11] Yu G, Gao J, Hummelen J C. Polymer photovoltaic cells: enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science, 1995, 270, 1789 doi: 10.1126/science.270.5243.1789[12] Bin H, Gao L, Zhang Z G, et al. 11.4% efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor. Nat Commun, 2016, 7, 13651 doi: 10.1038/ncomms13651[13] Sun C, Pan F, Bin H, et al. A low cost and high performance polymer donor material for polymer solar cells. Nat Commun, 2018, 9, 743 doi: 10.1038/s41467-018-03207-x[14] Qian D, Ye L, Zhang M, et al. Design, application, and morphology study of a new photovoltaic polymer with strong aggregation in solution state. Macromolecules, 2012, 45, 9611 doi: 10.1021/ma301900h[15] Zhang M, Guo X, Ma W, et al. A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. Adv Mater, 2015, 27, 4655 doi: 10.1002/adma.201502110[16] Zhang S, Qin Y, Zhu J, et al. Over 14% efficiency in polymer solar cells enabled by a chlorinated polymer donor. Adv Mater, 2018, 30, 1800868 doi: 10.1002/adma.201800868[17] Huo L, Liu T, Sun X, et al. Single-junction organic solar cells based on a novel wide-bandgap polymer with efficiency of 9.7%. Adv Mater, 2015, 27, 2938 doi: 10.1002/adma.201500647[18] Liu T, Huo L, Chandrabose S, et al. Optimized fibril network morphology by precise side-chain engineering to achieve high-performance bulk-heterojunction organic solar cells. Adv Mater, 2018, 30, 1707353 doi: 10.1002/adma.201707353[19] Liang Y, Xu Z, Xia J, et al. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv Mater, 2010, 22, E135 doi: 10.1002/adma.200903528[20] Liao S H, Jhuo H J, Cheng Y S, et al. Fullerene derivative-doped zinc oxide nanofilm as the cathode of inverted polymer solar cells with low-bandgap polymer (PTB7-Th) for high performance. Adv Mater, 2013, 25, 4766 doi: 10.1002/adma.201301476[21] Price S C, Stuart A C, Yang L, et al. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer-fullerene solar cells. J Am Chem Soc, 2011, 133, 4625 doi: 10.1021/ja1112595[22] Hendriks K H, Heintges G H L, Gevaerts V S, et al. High-molecular-weight regular alternating diketopyrrolopyrrole-based terpolymers for efficient organic solar cells. Angew Chem, 2013, 125, 8499 doi: 10.1002/ange.201302319[23] Zhao J, Li Y, Yang G, et al. Efficient organic solar cells processed from hydrocarbon solvents. Nat Energy, 2016, 1, 15027 doi: 10.1038/nenergy.2015.27[24] Jin Y, Chen Z, Dong S, et al. A novel naphtho[1,2-c:5,6-c']bis([1,2,5]thiadiazole)-based narrow-bandgap π-conjugated polymer with power conversion efficiency over 10%. Adv Mater, 2016, 28, 9811 doi: 10.1002/adma.201603178[25] Fan B, Zhang D, Li M, et al. Achieving over 16% efficiency for single-junction organic solar cells. Sci China Chem, 2019, 62, 746 doi: 10.1007/s11426-019-9457-5[26] Liu Q, Jiang Y, Jin K, et al. 18% efficiency organic solar cells. Sci Bull, 2020, 65, 272 doi: 10.1016/j.scib.2020.01.001[27] Qin J, Zhang L, Zuo C, et al. A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency. J Semicond, 2021, 42, 010501 doi: 10.1088/1674-4926/42/1/010501[28] Cao J, Zhang W, Xiao Z, et al. Synthesis and photovoltaic properties of low band gap polymers containing benzo[1,2-b:4,5-c′]dithiophene-4,8-dione. Macromolecules, 2012, 45, 1710 doi: 10.1021/ma202578y[29] Zhang W, Cao J, Liu Y, et al. Using cyclopenta[2,1-b:3,4-c']dithiophene-4-one as a building block for low-bandgap conjugated copolymers applied in solar cells. Macromol Rapid Commun, 2012, 33, 1574 doi: 10.1002/marc.201200311[30] Cao J, Liao Q, Du X, et al. A pentacyclic aromatic lactam building block for efficient polymer solar cells. Energy Environ Sci, 2013, 6, 3224 doi: 10.1039/c3ee41948g[31] Cao J, Zuo C, Du B, et al. Hexacyclic lactam building blocks for highly efficient polymer solar cells. Chem Commun, 2015, 51, 12122 doi: 10.1039/C5CC04375A[32] Cao J, Qian L, Lu F, et al. A lactam building block for efficient polymer solar cells. Chem Commun, 2015, 51, 11830 doi: 10.1039/C5CC03620H[33] An M, Xie F, Geng X, et al. A high-performance D-A copolymer based on dithieno[3,2-b:2′,3′-d]pyridin-5(4H)-one unit compatible with fullerene and nonfullerene acceptors in solar cells. Adv Energy Mater, 2017, 7, 1602509 doi: 10.1002/aenm.201602509[34] Lin Y, Wang J, Zhang Z G, et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv Mater, 2015, 27, 1170 doi: 10.1002/adma.201404317[35] Li D, Xiao Z, Wang S, et al. A thieno[3,2-c]isoquinolin-5(4H)-one building block for efficient thick-film solar cells. Adv Energy Mater, 2018, 8, 1800397 doi: 10.1002/aenm.201800397[36] Gao Y, Li D, Xiao Z, et al. High-performance wide-bandgap copolymers with dithieno[3,2-b:2′,3′-d]pyridin-5(4H)-one units. Mater Chem Front, 2019, 3, 399 doi: 10.1039/C8QM00604K[37] Li S, Ye L, Zhao W, et al. Energy-level modulation of small-molecule electron acceptors to achieve over 12% efficiency in polymer solar cells. Adv Mater, 2016, 28, 9423 doi: 10.1002/adma.201602776[38] Liu J, Liu L, Zuo C, et al. 5H-dithieno[3,2-b:2′,3′-d]pyran-5-one unit yields efficient wide-bandgap polymer donors. Sci Bull, 2019, 64, 1655 doi: 10.1016/j.scib.2019.09.001[39] Yuan J, Zhang Y, Zhou L, et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule, 2019, 3, 1140 doi: 10.1016/j.joule.2019.01.004[40] Xiong J, Jin K, Jiang Y, et al. Thiolactone copolymer donor gifts organic solar cells a 16.72% efficiency. Sci Bull, 2019, 64, 1573 doi: 10.1016/j.scib.2019.10.002[41] Jiang K, Wei Q, Lai J Y L, et al. Alkyl chain tuning of small molecule acceptors for efficient organic solar cells. Joule, 2019, 3, 3020 doi: 10.1016/j.joule.2019.09.010[42] Li W, Liu Q, Jin K, et al. Fused-ring phenazine building blocks for efficient copolymer donors. Mater Chem Front, 2020, 4, 1454 doi: 10.1039/D0QM00080A[43] Xiong J, Xu J, Jiang Y, et al. Fused-ring bislactone building blocks for polymer donors. Sci Bull, 2020, 65, 1792 doi: 10.1016/j.scib.2020.07.018[44] Wang Z, Peng Z, Xiao Z, et al. Thermodynamic properties and molecular packing explain performance and processing procedures of three D18:NFA organic solar cells. Adv Mater, 2020, 32, 2005386 doi: 10.1002/adma.202005386[45] Qin J, Zhang L, Xiao Z, et al. Over 16% efficiency from thick-film organic solar cells. Sci Bull, 2020, 65, 1979 doi: 10.1016/j.scib.2020.08.027 -
Supplements
 20120028suppl.pdf
20120028suppl.pdf

-
Proportional views





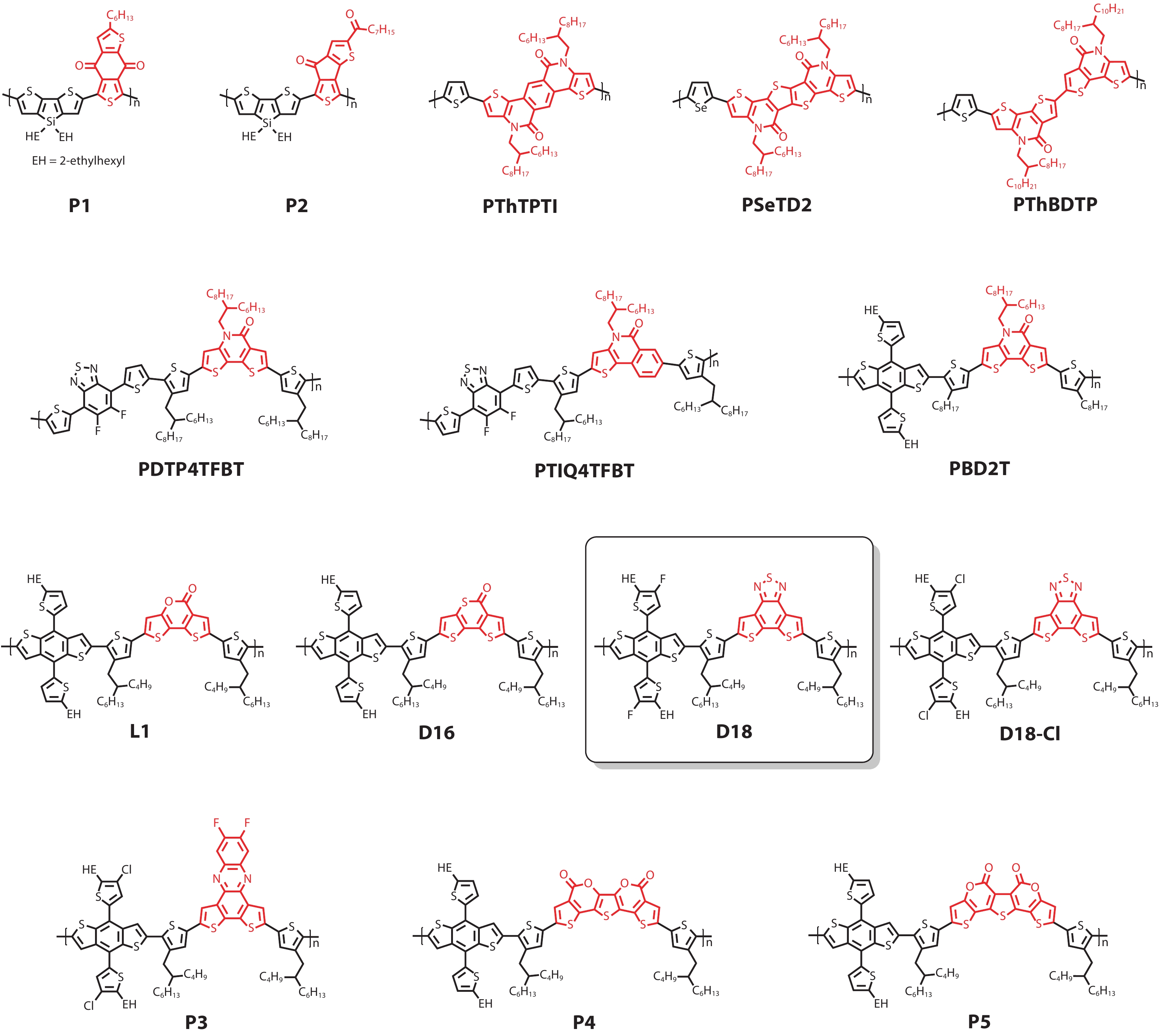
 DownLoad:
DownLoad: