| Citation: |
Zhitao Han, Sisi Li, Jinkui Chu, Yong Chen. Controlled growth of well-aligned ZnO nanowire arrays using the improved hydrothermal method[J]. Journal of Semiconductors, 2013, 34(6): 063002. doi: 10.1088/1674-4926/34/6/063002
****
Z T Han, S S Li, J K Chu, Y Chen. Controlled growth of well-aligned ZnO nanowire arrays using the improved hydrothermal method[J]. J. Semicond., 2013, 34(6): 063002. doi: 10.1088/1674-4926/34/6/063002.
|
Controlled growth of well-aligned ZnO nanowire arrays using the improved hydrothermal method
DOI: 10.1088/1674-4926/34/6/063002
More Information
-
Abstract
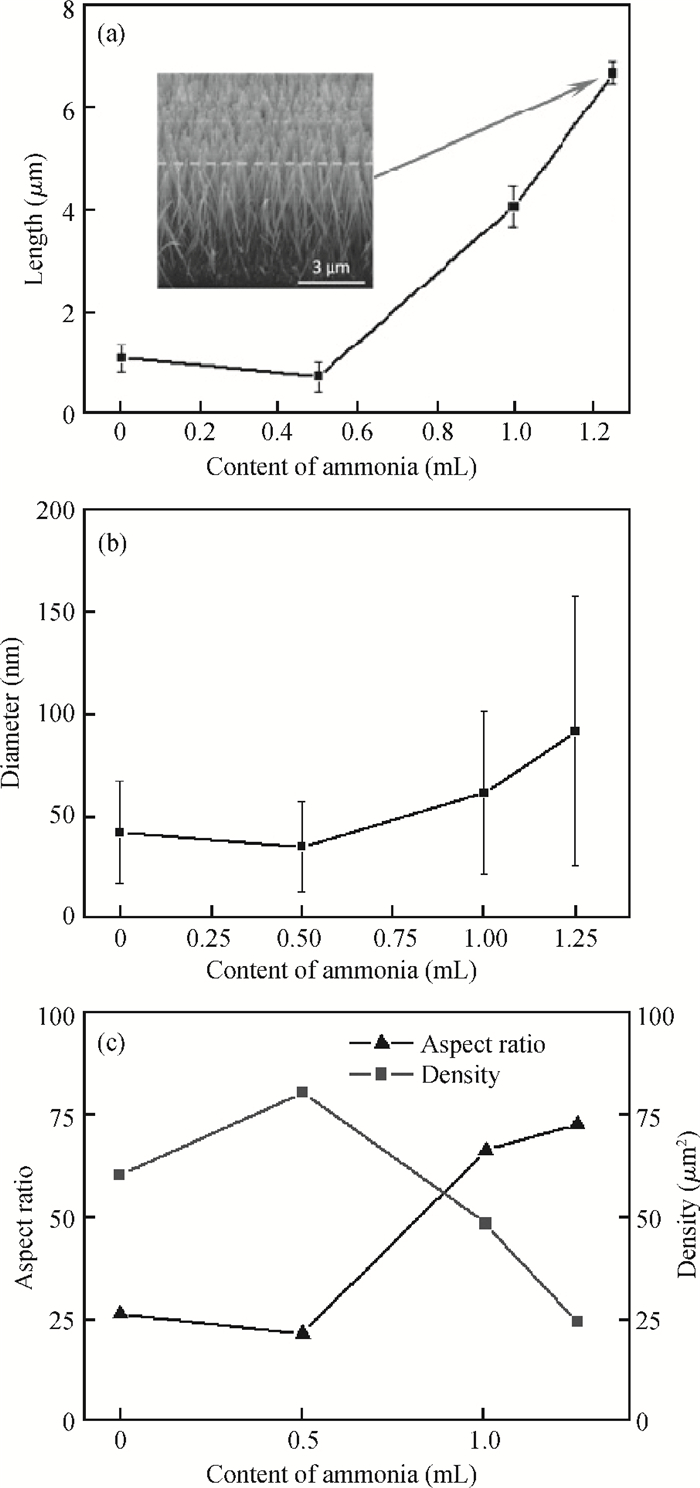
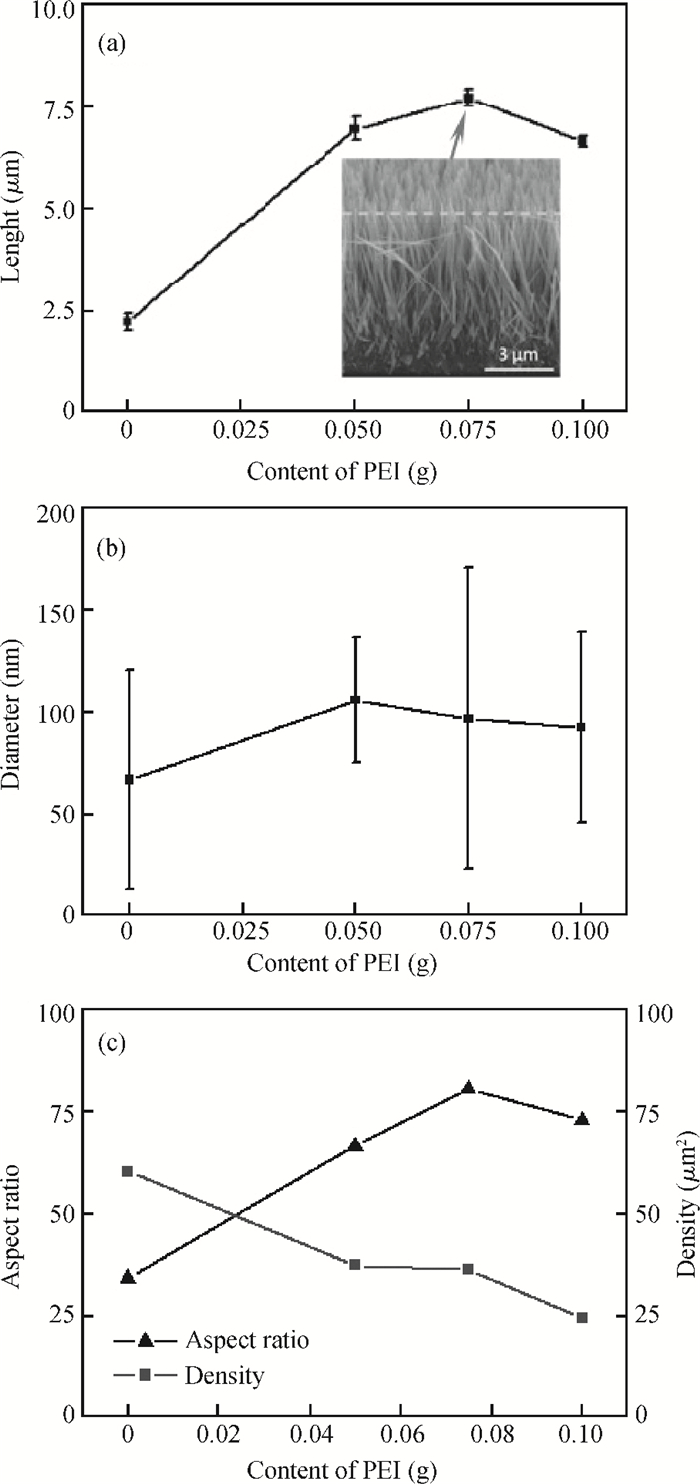
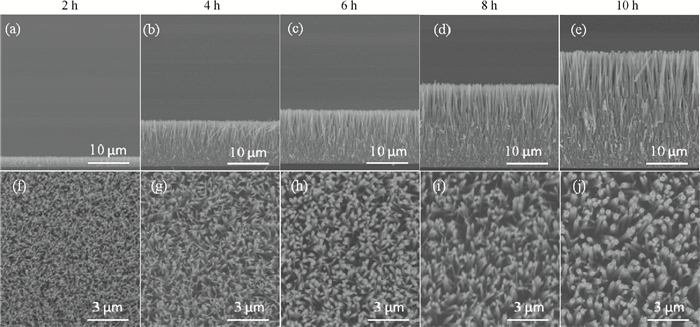
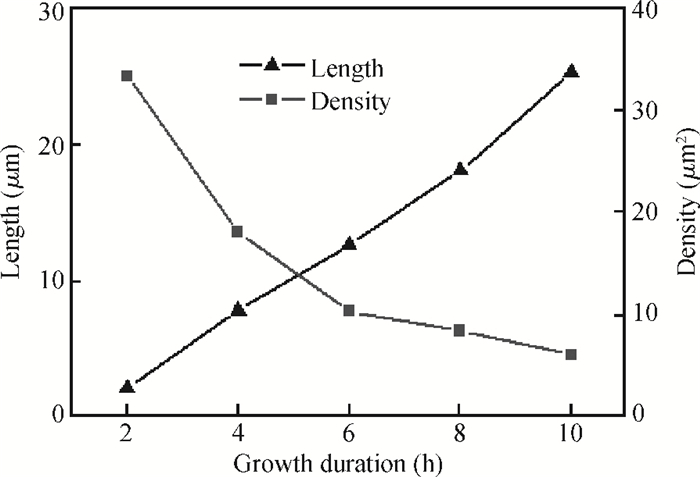
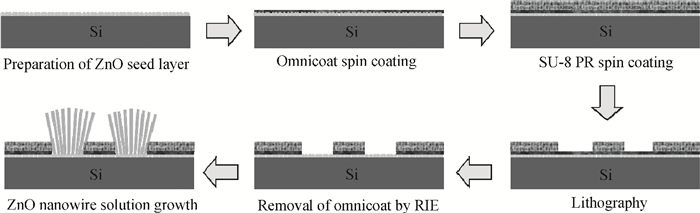
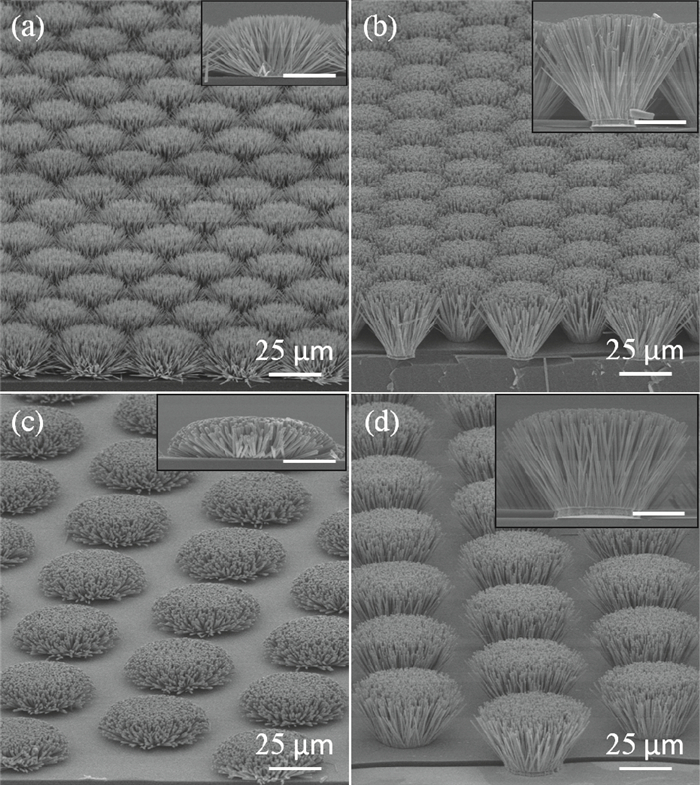
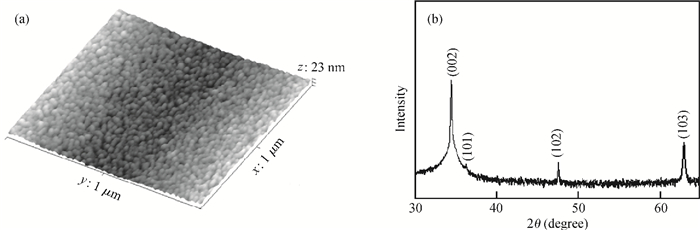
Well-aligned ZnO nanowires were hydrothermally synthesized based on a facile method for preparing the ZnO seed layer which was derived from the combination of a sol-gel process and the spin-coating technique. The effect of the contents of growth solution and the growth duration on the morphology of ZnO nanowires has been investigated. The results indicated that long and vertically aligned ZnO nanowires could be obtained by adjusting the contents of ammonia and polyethyleneimine (PEI) in the growth solution. Under the optimized condition, the length of ZnO nanowires increased fast and almost linearly with the growth duration. After 10 h incubation, ZnO nanowires more than 25 μm in length were obtained. By combining the conventional photolithographic method with this hydrothermal approach, long and well-aligned ZnO nanowire arrays were selectively grown on the substrate. In addition, the bottom fusion at the foot of the nanowires has been obviously improved. The results demonstrated that the improved hydrothermal process is favorable to synthesize long and well-aligned ZnO nanowires, and possesses good process compatibility with the conventional photolithographic technique for preparing ZnO nanowire arrays. So it has great potential in applications such as display and field emission devices.-
Keywords:
- ZnO,
- nanowires,
- hydrothermal,
- photolithography
-
References
[1] Han X G, He H Z, Kuang Q, et al. Controlling morphologies and tuning the related properties of nano/microstructured ZnO crystallites. J Phys Chem C, 2009, 113(2):584 doi: 10.1021/jp808233e[2] Law M, Greene L E, Johnson J C, et al. Nanowire dye-sensitized solar cells. Nature Mater, 2005, 4(6):455 doi: 10.1038/nmat1387[3] Chu D, Masuda Y, Ohji T, et al. Formation and photocatalytic application of ZnO nanotubes using aqueous solution. Langmuir, 2010, 26(4):2811 doi: 10.1021/la902866a[4] Jun J H, Seong H, Cho K, et al. Ultraviolet photodetectors based on ZnO nanoparticles. Ceramics International, 2009, 35(7):2797 doi: 10.1016/j.ceramint.2009.03.032[5] Chen H, Liu Y, Xie C S, et al. A comparative study on UV light activated porous TiO2 and ZnO film sensors for gas sensing at room temperature. Ceramics International, 2012, 38(1):503 doi: 10.1016/j.ceramint.2011.07.035[6] Huang M H, Mao S, Feick H, et al. Room-temperature ultraviolet nanowire nanolasers. Science, 2001, 292(5523):1897 doi: 10.1126/science.1060367[7] Zhang Y A, Wu C X, Zheng Y, et al. Synthesis and efficient field emission characteristics of patterned ZnO nanowires. Journal of Semiconductors, 2012, 33(2):023001 doi: 10.1088/1674-4926/33/2/023001[8] Li S Y, Lee C Y, Tseng T Y, et al. Copper-catalyzed ZnO nanowires on silicon (100) grown by vapor-liquid-solid process. J Cryst Growth, 2003, 247(3/4):357 https://ir.nctu.edu.tw/handle/11536/28228?locale=en[9] Deng H, Tang B, Cheng H, et al. Horizontal growth and ultraviolet sensitivity characteristics of ZnO nanowires on sapphire substrates. Chinese Journal of Semiconductors, 2007, 28(1):56 http://www.oalib.com/paper/1522959[10] Lee D J, Park J Y, Yun Y S, et al. Comparative studies on the growth behavior of ZnO nanorods by metal organic chemical vapor deposition depending on the type of substrates. J Cryst Growth, 2005, 276(3/4):458[11] Premkumar T, Zhou Y S, Lu Y F, et al. Optical and field-emission properties of ZnO nanostructures deposited using high-pressure pulsed laser deposition. J Appl Mater Interfaces, 2010, 2(10):2863 doi: 10.1021/am100539q[12] Tian J H, Hu J, Li S S, et al. Improved seedless hydrothermal synthesis of dense and ultralong ZnO nanowires. Nanotechnology, 2011, 22:245601 doi: 10.1088/0957-4484/22/24/245601[13] Zhang L L, Guo C X, Chen J G, et al. Solution growth of morphology controllable ZnO one-dimensional nanorods and microrods. Chinese Journal of Semiconductors, 2005, 26(11):2127[14] Xu S, Lao C S, Weintraub B, et al. Density-controlled growth of aligned ZnO nanowire arrays by seedless chemical approach on smooth surfaces. J Mater Res, 2008, 23(8):2072 doi: 10.1557/JMR.2008.0274[15] Greene L E, Law M, Goldberger J, et al. Low-temperature wafer-scale production of ZnO nanowire arrays. Angew Chem Int Ed, 2003, 42(26):3031 doi: 10.1002/anie.200351461[16] Greene L E, Law M, Tan D H, et al. General route to vertical ZnO nanowire arrays using textured ZnO seeds. Nano Lett, 2005, 5(7):1231 doi: 10.1021/nl050788p[17] Kanjwal M A, Sheikh F A, Barajat N A M, et al. Zinc oxide's hierarchical nanostructure and its photocatalytic properties. Appl Surf Sci, 2012, 258(8):36953702 http://orbit.dtu.dk/files/6497014/science%5B1%5D.pdf[18] Li Y H, Gong J, Deng Y L. Hierarchical structured ZnO nanorods on ZnO nanofibers and their photoresponse to UV and visible lights. Sensors and Actuators A, 2010, 158(2):176 doi: 10.1016/j.sna.2009.12.030[19] Park J Y, Kim S S. Growth of nanograins in electrospun ZnO nanofibers. J Am Ceram Soc, 2009, 92(8):1691 doi: 10.1111/jace.2009.92.issue-8[20] Xu C K, Shin P, Cao L L, et al. Preferential growth of long ZnO nanowire array and its application in dye-sensitized solar cells. J Phys Chem C, 2010, 114(1):125 doi: 10.1021/jp9085415[21] Kartawidjaja F C, Lim Z Y, Ng S L G, et al. Morphology, optical, and magnetic properties of Zn1-xCoxO nanorods grown via a wet chemical route. J Am Ceram Soc, 2009, 93(11):3798[22] Strom J G, Jun H W. Kinetics of hydrolysis of methenamine. J Pharm Sci, 1980, 69(11):1261 doi: 10.1002/jps.2600691107[23] Govender K, Boyle D S, Kenway P B, et al. Understanding the factors that govern the deposition and morphology of thin films of ZnO from aqueous solution. J Mater Chem, 2004, 14:2575 doi: 10.1039/B404784B[24] Greene L E, Yuhas B D, Law M, et al. Solution-grown zinc oxide nanowires. Inorg Chem, 2006, 45(19):7535 doi: 10.1021/ic0601900[25] Yamabi S, Imai H. Growth conditions for wurtzite zinc oxide films in aqueous solutions. J Mater Chem, 2002, 12:3773 doi: 10.1039/b205384e[26] Wu W B, Hu G D, Cui S G, et al. Epitaxy of vertical ZnO nanorod arrays on highly (001)-oriented ZnO seed monolayer by a hydrothermal route. Cryst Growth Des, 2008, 8(11):4014 doi: 10.1021/cg800210m -
Proportional views





 DownLoad:
DownLoad: