1. Introduction
Owing to its unique electrical properties such as low resistivity and high resistance to electromigration, copper (Cu) has taken the place of aluminum (Al) and become the main material for interconnection[1]. However, it is difficult for copper to associate with reactive ion etching (RIE), which is usually used in aluminum interconnection. To solve the problem, a Damascene structure is implemented to realize the copper on-chip interconnects. During the Damascene process, chemical mechanical polishing (CMP) is utilized to remove the overburdened copper, Ta/TaN barrier and so on, gaining an excellent surface planarity. Unfortunately, this process leaves a large amount of contamination on the wafer surface. The major contaminants include BTA, abrasive from the slurry, undesired metallic ions and other chemical components, all of which must be eliminated in the post-CMP cleaning process[2, 3].
Colloidal silica based slurry collocating with H2O2 is currently used in the Cu CMP process, due to its excellent ability to remove the tantalum layer; however, it also shows a strong tendency to adsorb colloidal silica on the copper surface, which accounts for most of the particles residual on the wafers polished[4, 5]. Moreover, the chemical components of slurry, the fragments of PVA brush and so on may also contribute to the residual particles. Those particles mentioned, if not removed completely, may cause a short circuit or open circuit after transferring the desired circuit, which eventually seriously influence the performance of the device or even result in its failure.
Many efforts have been devoted to the removal of particles, especially the colloidal silica abrasives from the polished copper surface[6, 7]. A surfactant is commonly used in post-CMP cleaning, since it can significantly reduce the surface tension of the liquid and interfacial tension, which is favorable to the particles removal. So a non-ionic surfactant is chosen as it cannot ionize in water and does not cause ion contamination. Experiments improved the adsorption state of particles, which can be controlled and changed with a non-ionic surfactant[8, 9].
In this study, a novel alkaline cleaning solution, namely FA/O cleaner, was developed for post-CMP cleaning. In order to enhance the efficiency of removing particles, the FA/O non-ionic surfactant, a kind of complex surfactant, was added in the cleaner. Therefore, the effect of the FA/O non-ionic surfactant was investigated in this paper.
2. Experiment
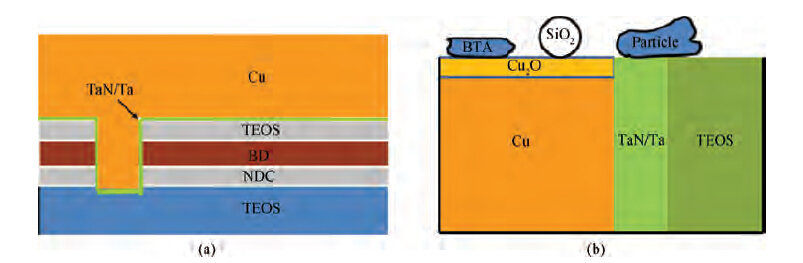
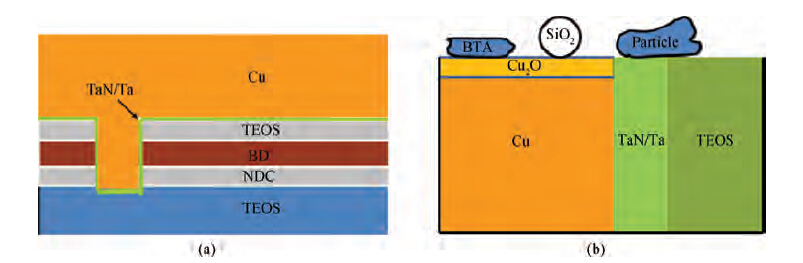
The CMP process was carried out on an Applied Materials 300 mm Reflexion LK tool, which was designed for a three-step copper CMP process and the requirement of dry in and dry out. Therefore, a three-step polishing scheme was used: step one was to remove bulk copper and gain an initially planarized surface; step two was to remove the residual copper and stop on barrier layers; and step three was to clean barrier metal and some of the dielectric. All the wafers were polished with the same Cu and barrier slurries. The slurry based on colloidal silica (14 wt %) with benzotriazole (BTA) and hydrogen peroxide (H2O2) was used in step three for barrier CMP. Subsequently, the post-CMP cleaning process was performed on the same machine. Firstly, the polished wafers were washed with megasonics. Then the wafers were cleaned in PVA brush box 1 and box 2. Finally, the wafers were blow-dried in an IPA vapor dryer. Once the whole process was completed, the 2825 broadband optical pattern wafer defect inspection tools (KLA Tencor) and SEMVisionTM G4 defect analysis platform (Applied Materials) were used to determine the cleaning performance. All the experiments were performed utilizing 12 inch copper pattern wafers. Figure 1(a) shows the schematic of pattern copper wafers, and Figure 1(b) shows the model of some residues on the wafer surface after the CMP process.
The cleaner chemical used in this study was provided by the Institute of Microelectronics, Hebei University of Technology, namely FA/O alkaline cleaner. In order to enhance the efficiency of particles removal, the FA/O non-ionic surfactant, which was also provided by the Institute of Microelectronics, was added in the cleaner. In this study, by varying the concentration of the non-ionic surfactant, a series of experiments were projected to estimate the effect of the non-ionic surfactant in the post CMP-cleaning process. The dilution ratios of the non-ionic surfactant, which were altered in the experiment, are listed in Table 1 and the other parameters for PVA brush scrubbing are fixed below: the brush rotation speed is 700 rpm, the DIW flow rate is 3200 mL/min and the cleaning time is 60~s.
3. Results and discussion
3.1 Effect of surfactant
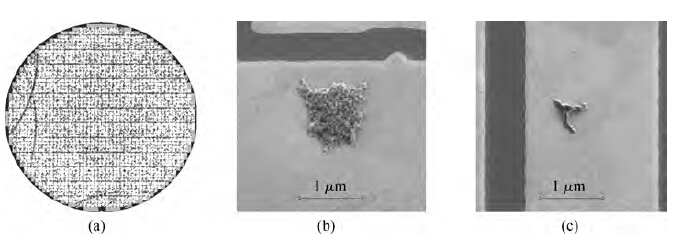
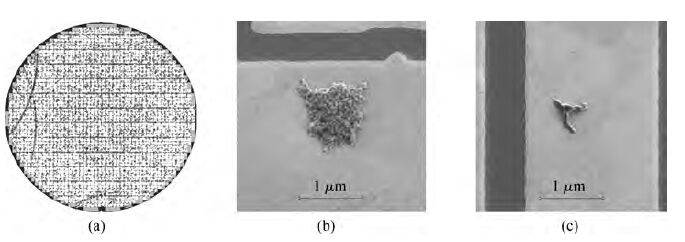
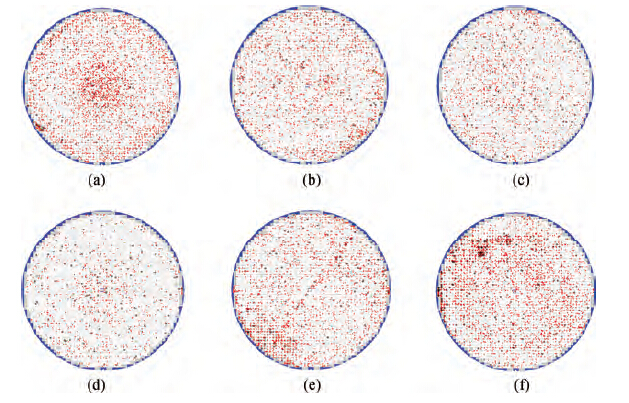
Figure 2(a) shows the single defect map of the pattern wafer processed with Cleaner A without the non-ionic surfactant. There are 8064 defects on this map; the defects include aspects such as BTA, particle, colloidal silica abrasive, and organic contamination, whose sizes are larger than 0.2 μm. Then one hundred region spots were selected randomly to determine what the defects really were. It was observed that more than half of the defects were colloidal silica abrasives and organic particles residual as shown in Figure 2(b).
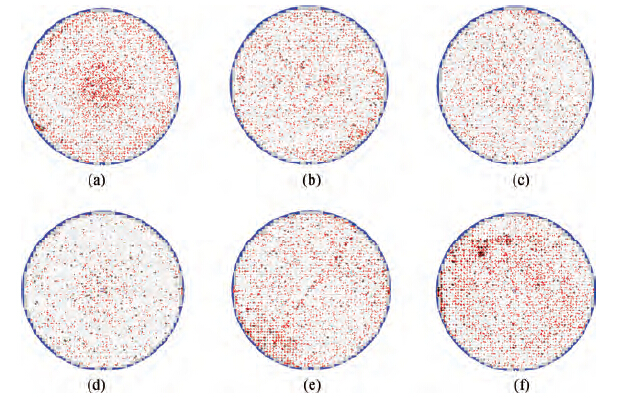
Figure 3 shows the single defect maps of the wafers treated with cleaners B, C, D, E, F and G. With the surfactant concentration increasing, fewer and fewer defects were found on the wafers. However, as shown in Figures 3(e) and 3(f), worse surfaces occurred on the wafers washed with Cleaner F and Cleaner G, which had higher concentrations of non-ionic surfactant. There are much fewer defects on the wafers treated with Cleaner E than Cleaner A, as shown in Figure 3(d). Moreover, only one or two colloidal silica abrasive were confirmed on the different wafers, all of which were washed with Cleaner E. The experiment result shows the great performance of the non-ionic surfactant on particle removal. It shows a trend that when the dilution rate is smaller than 1/75, the bigger the dilution rate is, the better the post-CMP cleaning results are; otherwise, the post-CMP cleaning results deteriorate with the concentration of the non-ionic surfactant further increasing.
3.2 Effect of surfactant on the removal of colloidal silica abrasives
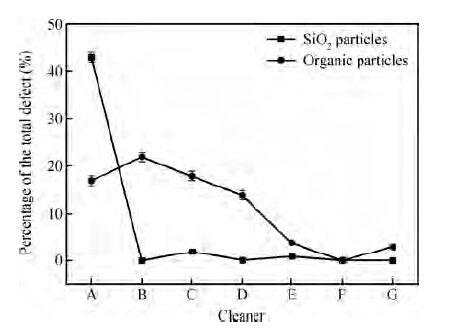
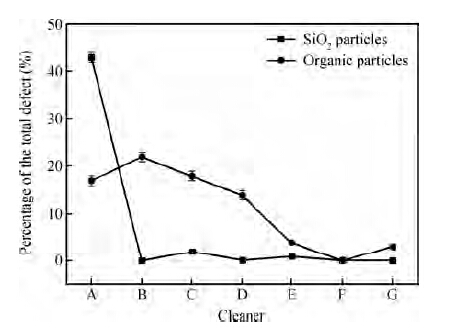
Figure 4 shows the percentage of colloidal silica abrasives and organic particles on wafers treated with different cleaners. From Figure 4, we can see that the colloidal silica abrasives are perfectly removed even at a low concentration of FA/O non-ionic surfactant.
The mechanism of the colloidal silica abrasive adsorbed on the wafer surface may be categorized as follows.
(1) The gravitation such as the Vander Waals force and electrostatic force existing between the wafer and the colloidal abrasives nearby makes these abrasives be adsorbed on the wafers rapidly, namely the physical absorption state.
(2) With H2O2 existing in the barrier slurry, copper tends to be oxidized to form cupric/cupreous oxides (CuO or Cu2O) and hydroxides passivation on wafer surface, which may result in the silica abrasives being chemisorbed onto this oxide layer by means of oxygen bridging bonding, namely the chemical adsorption state.
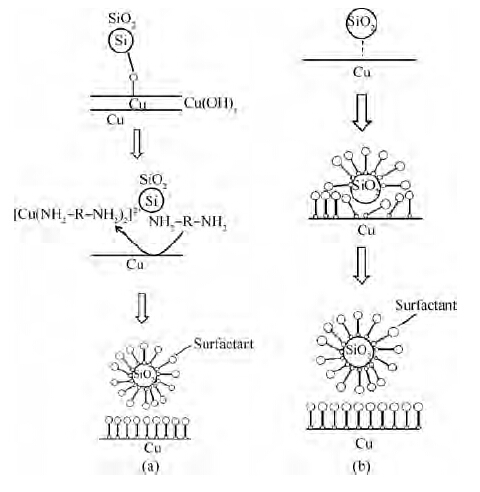
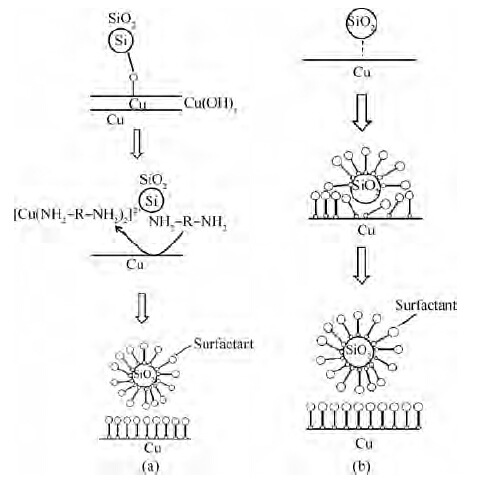
One of the effective components of the FA/O alkaline cleaner is the FA/O chelating agent. The FA/O chelating agent is a potent alkaline chelating agent with 13 chelate rings. It can react with mental ions to give a stable and soluble chelate-metal complex. As for the FA/O non-ionic surfactant, it is a kind of complex surfactant consisting of an osmotic agent and a dispersant. A schematic illustrating how the FA/O cleaner with the non-ionic surfactant removes the colloidal silica abrasives is shown in Figure 5. In the removal of chemical adsorbed colloidal silica particles, the FA/O chelating agent plays an important role. As Figure 5(a) shows, the chelating agent firstly reacts with Cu ions to give a stable and soluble chelate-metal complex[10]:
|
CuO2+O2→CuO+H2OOH−→Cu(OH)2⟺Cu2++2OH−, |
(1) |
|
Cu2++2NH2−R−NH2→[Cu(NH2−R−NH2)2]2+. |
(2) |
Thus a thin surface layer of copper oxides is removed and the attached colloidal silica is released. According to the preferential adsorption theory, the surfactant molecule is adsorbed on the wafer surface to decrease the new surface energy, forming a molecule layer on the wafer surface to prevent the particle from being re-adsorbed on the wafer surface.
As for the physically adsorbed colloidal silica abrasives, the osmotic agent molecule that the surfactant contains can permeate and expand between the wafer and the colloidal silica abrasives, as shown in Figure 5(b). The dispersant molecule holds up the colloidal silica particles and replaces it on the wafer surface. At the same time, the silica is surrounded by a layer of complex surfactant molecules, as shown in Figures 5(a) and 5(b). Once desorbed from the wafer surface, the colloidal silica abrasives are easily removed by the PVA brush and washed away with the flow fluid.
From Figure 4, we can also see that the higher the concentration of the non-ionic surfactant is, the more efficient the organic particles removal is. Since each wafer underwent the cleaning process immediately after polishing, it is hard for organic particles to form chemical bonding with the wafer surface; that is to say, most of the organic particles were in the physical absorption state. So the mechanism of how the organic particles are removed is similar to the physically adsorbed colloidal silica abrasives, as Figure 5(b) shows.
3.3 Negative effect of surfactant
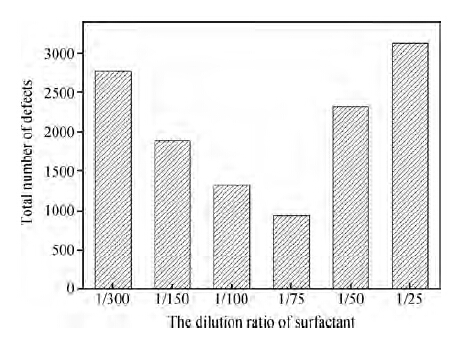
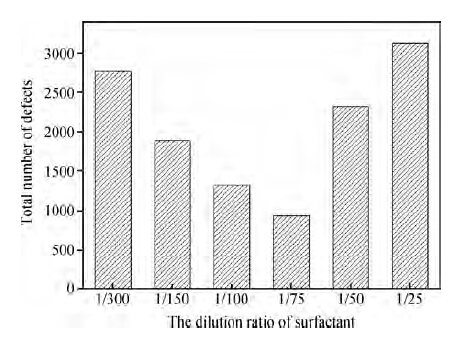
As mentioned above, the worst results occurred on wafers where the dilution ratio of the surfactant was bigger than 1/75. Figure 7 gives the total number of defects for different ratios of non-ionic surfactant. It shows that when the dilution rate is bigger than 1/75, the post-CMP cleaning results gets worse and worse with the increasing concentration of surfactant.
Further detection indicated that the organic contamination, as shown in Figure 8, accounted for nearly 25 % of the defects on the wafers after cleaning with cleaners F and G, which led to the increase of the defects. As shown in Figure 5, the non-ionic surfactant can form a protective layer of film on the wafer surface to enhance the removal of particles. However, this layer of protective film itself belongs to organic contamination. The molecule of the surfactant is large, so it can be adsorbed on the wafer, which is also easy to remove. Even so, there is still some organic contamination residue on the wafer surface after cleaning. With the concentration of non-ionic surfactant increasing, more and more organic contamination defects are left on the wafer. When the concentration of surfactant is bigger than a certain value, such as 1/75, the increase of organic contamination exceeds the decrease of other defects, finally resulting in the total defects increasing.
4. Conclusions
With non-ionic surfactant taking part in the post-CMP cleaning process, the cleaning efficiency is improved and better cleaning results are obtained. The non-ionic surfactant has a great performance on the removal of colloidal silica abrasives and organic particles through chemical and mechanical reaction. What is more, the non-ionic surfactant can improve the surface roughness by removing the particles and decreasing scratching. However, the non-ionic surfactant also brings about organic contamination, for it can be adsorbed on the wafer surface. It is concluded that there is a certain value for the concentration of non-ionic surfactant with which the wafer gets the minimum defects after cleaning.




 DownLoad:
DownLoad:
 DownLoad:
DownLoad:











 DownLoad:
DownLoad:










