| Citation: |
Wenguo Ning, Heng Li, Chunsheng Zhu, Le Luo, Dong Chen, Zhenzhen Duan. The effects of cure temperature history on the stability of polyimide films[J]. Journal of Semiconductors, 2013, 34(6): 063003. doi: 10.1088/1674-4926/34/6/063003
****
W G Ning, H Li, C S Zhu, L Luo, D Chen, Z Z Duan. The effects of cure temperature history on the stability of polyimide films[J]. J. Semicond., 2013, 34(6): 063003. doi: 10.1088/1674-4926/34/6/063003.
|
The effects of cure temperature history on the stability of polyimide films
DOI: 10.1088/1674-4926/34/6/063003
More Information
-
Abstract
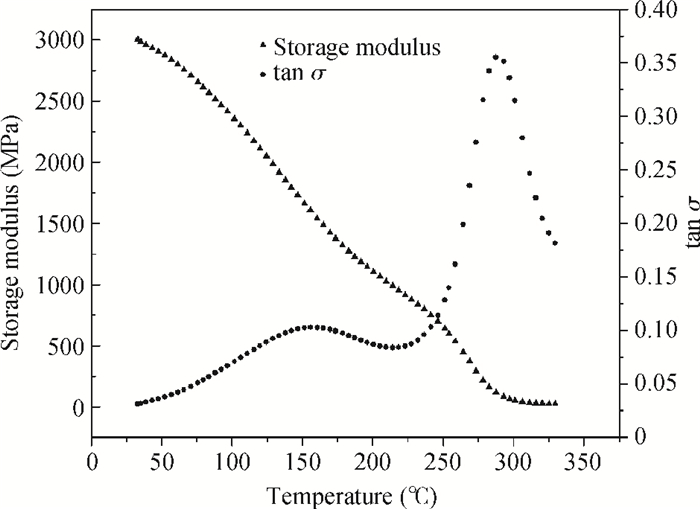
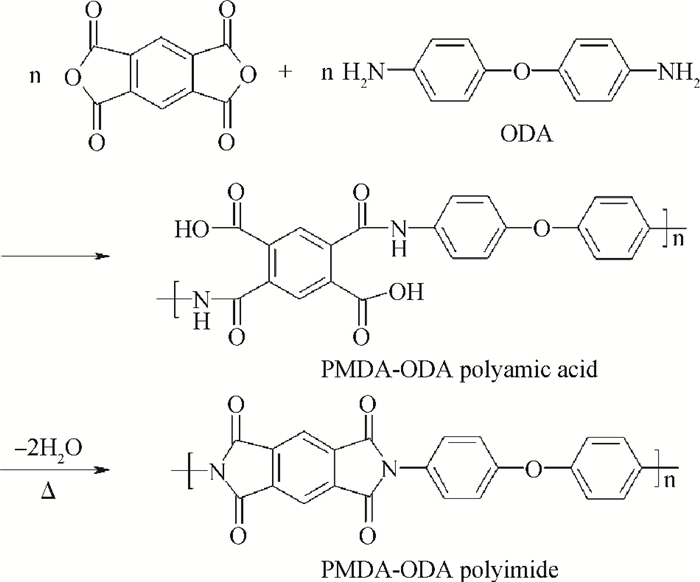
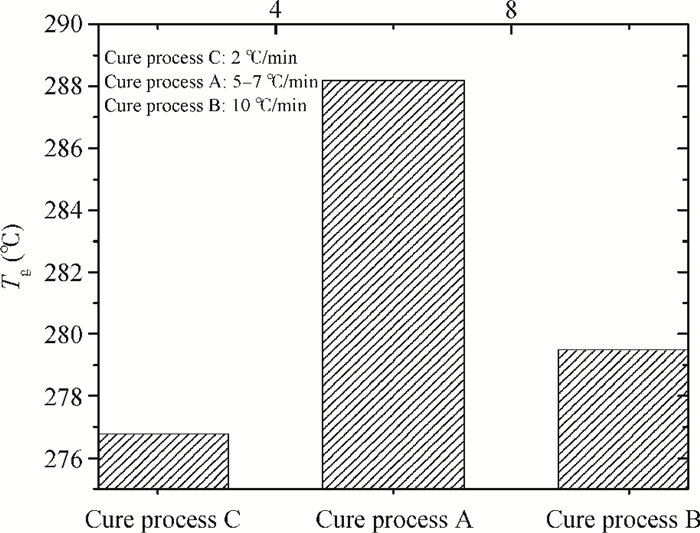
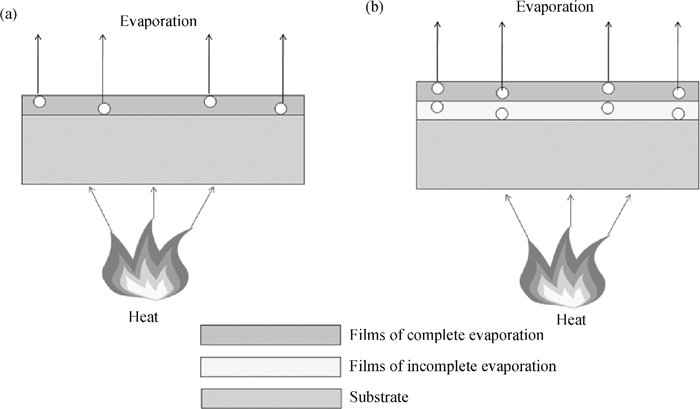
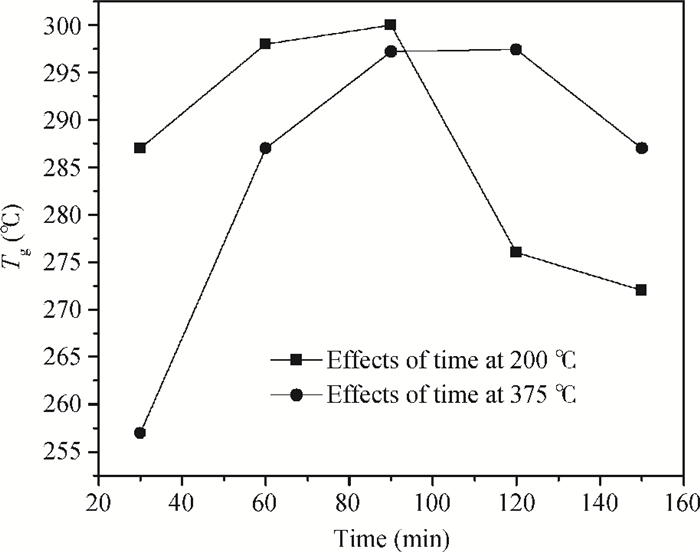
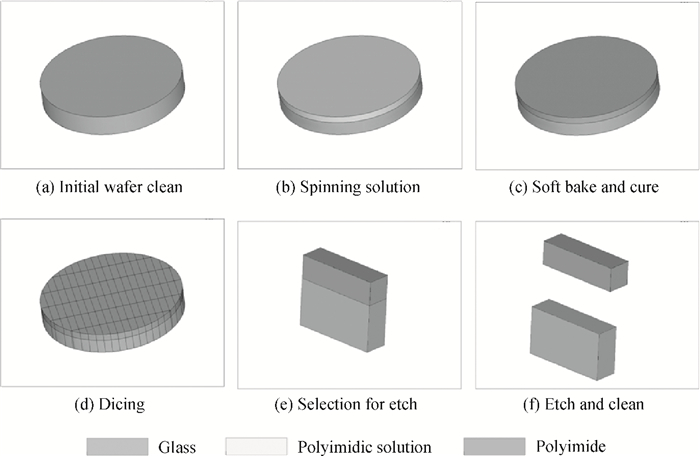
The effects of cure temperature history on the stability of hinged structure poly (4, 4-oxydiphenylene pyromellitimide) (PMDA-ODA) polyimide were studied by dynamic mechanical analysis. The polyimide films were cured under different curing conditions and peeled off by substrate etching. It was found that a proper cure time and temperature ramp rate improves the stability in terms of higher glass transition temperature. Ninety minutes at 375℃ or 200℃ is a beneficial high glass transition temperature. The temperature ramp rate should be between 2℃/min and 10℃/min, which is neither too high nor too low.-
Keywords:
- curing of polymers,
- polyimide,
- stabilization,
- DMA
-
References
[1] Russell J D, Kardos J L. Crosslinking characterization of a polyimide:AFR700B. Polymer Composites, 1997, 18(5):595 doi: 10.1002/(ISSN)1548-0569[2] Rich D C, Sichel E K, Cebe P. Curing study of a preimidized photosensitive polyimide. Polymer Eng Sci, 1996, 36(17):2179 doi: 10.1002/(ISSN)1548-2634[3] Sasaki T, Yokota R. Synthesis and properties of an addition-type imide oligomer having pendent phenylethynyl groups:investigation of curing behavior. High Performance Polymers, 2006, 18(2):199 doi: 10.1177/0954008306058269[4] Kang P H, Jeon Y K, Jeun J P, et al. Effect of electron beam irradiation on polyimide film. Journal of Industrial and Engineering Chemistry, 2008, 14(5):672 doi: 10.1016/j.jiec.2008.03.004[5] Artiaga R, Chipara M, Stephens C P, et al. Dynamical mechanical analysis of proton beam irradiated polyimide. Nuclear Instruments and Methods in Physics Research Section B:Beam Interactions with Materials and Atoms, 2005, 236(1-4):432 doi: 10.1016/j.nimb.2005.04.013[6] Chen C, Qin W, Huang X. Synthesis and characterization of novel polyimides derived from 1, 4-bis (4-aminophenoxymethylene) cyclohexane (BAMC) and two aromatic dianhydrides. Journal of Macromolecular Science, Part B, 2008, 47(4):783 doi: 10.1080/00222340802119273[7] Li F, Ge J J, Honigfirt P S, et al. Dianhydride architectural effects on the relaxation behaviors and thermal and optical properties of organo-soluble aromatic polyimide films. Polymer, 1999, 40(18):4987 doi: 10.1016/S0032-3861(98)00721-6[8] Mircea I C. On an anomalous kinetic in irradiated polymers around the glass transition temperature. Nuclear Instruments and Methods in Physics Research Section B:Beam Interactions with Materials and Atoms, 1997, 131(1-4):180 doi: 10.1016/S0168-583X(97)00340-6[9] Chipara M, Benson R, Chipara M, et al. ESR investigations on irradiated polystyrene. Nuclear Instruments and Methods in Physics Research Section B:Beam Interactions with Materials and Atoms, 2003, 208(0):390 http://cat.inist.fr/?aModele=afficheN&cpsidt=14952935[10] Pireaux J J, Gregoire C, Caudano R, et al. Electron-induced vibrational spectroscopy——a new and unique tool to unravel the molecular-structure of polymer surfaces. Langmuir, 1991, 7(11):2433 doi: 10.1021/la00059a006[11] Chern Y T, H C Shiue. High subglass transition temperatures and low dielectric constants of polyimides derived from 4, 9-bis (4-aminophenyl) diamantane. Chem Mater, 1998, 10(1):210 doi: 10.1021/cm970341k[12] American Society of Metals. Characterization and failure analysis of plastics. Ohio:ASM International, 2003:119 http://cityofmaterials.asminternational.org/content/ASM/StoreFiles/06978G_Frontmatter.pdf[13] Gowariker V R, Viswanathan N V, Sreedhar J. Polymer science. New York:John Wiley & Sons 1986:164[14] Fox T G, Flory P J. The glass temperature and related properties of polystyrene:influence of molecular weight. J Polymer Sci, 1954, 14(75):315 doi: 10.1002/pol.1954.120147514 -
Proportional views





 DownLoad:
DownLoad: