1. Introduction
As is well known the structure and properties of the organic semiconductor materials are highly dependent upon their processing technology[1, 2, 3]. Generally, the organic materials have a large molecular weight, strong intramolecular and weak Van der Waal's intermolecular bondings. For this reason organic semiconductors are found to be suitable for centrifugal processing and this technique has shown interesting results while investigation has been performed with different kinds of organic materials[4, 5, 6]. Ahmed et al.[7] fabricated the heterojunctions using centrifugation by employing p-type Si and the thin films of the poly-N-epoxypropylcarbazole (PEPC) doped with tetracyanoquinodimethane (TCNQ). The PEPC films were grown on Si wafers at room temperature under different gravity conditions, i.e. 1, 123, 277 and 1107 g. It was observed that the grown organic polymer films were uniformly spread over the substrate with good adhesion. Current-voltage (I-V) characteristics of the grown hybrid structures were evaluated as a function of temperature ranging from 20 to 60 C. It was found that all samples are p-p isotype heterojunctions. Rectification ratio, threshold voltage, reverse saturation current and junction resistance of the fabricated junctions were evaluated at different temperatures. In Reference [8] a number of organo-metallic vanadium complexes such as VO2(3-fl), VO(PBO)2 and VO(DBM) were synthesized and their thin films were deposited from solutions in organic solvent at different gravity conditions, i.e. 183 g, 733 g and 1650 g by centrifugation. In the result Al/VO2 (3-fl)/Ga, Cu/VO2 (3-fl)/Ga, Al/Vanadyl (acac)/Ga, Cu/Vanadyl (acac)/Ga, Al/VO(PBO)2/Ga, Cu/VO(PBO)2/Ga, Al/VO(DBM)/Ga, Cu/VO(DBM)/Ga, Al/Vanadyl (acac)/TiO2/Ga and Cu/VO2(3-fl)/TiO2/Ga sandwich-type samples were fabricated. Investigation of I-V characteristics of the samples was carried out and it was found that all samples show non-linear rectification behavior. In VO2 (3-fl) and Vanadyl (acac) films switching effect was observed from low-conductance to high-conductance states and write-once-read-many-times (WORM) memory effect has also been observed in the case of the samples with Cu electrodes.
Keeping in mind the potential of the centrifugation technique for electronics applications, in this study, we used orange-dye (OD) as an active material along with its composite with carbon nanotubes (CNTs). The humidity sensing properties of OD have already been studied by Moiz et al.[9]. The OD is reported as a p-type semiconductor material and it is referred to as an environment friendly organic material[10, 11]. In Reference [11], humidity sensors by drop-casting were fabricated by the deposition of OD-PANI composite films from the blend of orange dye and PANI in distilled water. The capacitance and impedance of the sensors were measured in the range of humidity of 30 %-90 %. The impedance of the sensor decreases exponentially, but capacitance increases with an increase in humidity. During humidification and dehumidification, in the case of thick OD films, the hysteresis was observed. The studies on the OD show that the OD could be a potential organic material to measure the environmental humidity in the specific RH range.
In this paper, in continuation of our efforts at investigation of humidity sensors and high gravity materials processing, we are presenting the data on the deposition of orange dye and orange dye-CNT composite thin films at high gravity conditions by centrifugation and humidity sensing electrical properties of the films.
2. Experimental
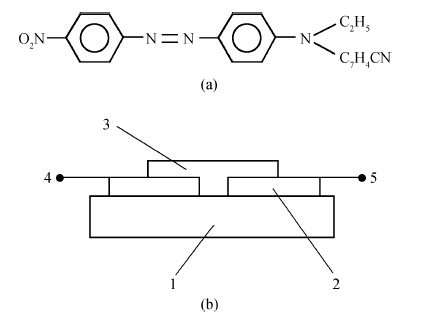
The thin films were deposited from the aqueous solution of orange dye (5 wt. %) and of its composites with CNTs (5 wt. %) at 1 g and 70 g gravity conditions. At 1 g the films were deposited by drop-casting and at 70 g by centrifugation in a tabletop centrifuge HETT ICH EBA-20 S. Acceleration (a) was calculated by the relation a=Rω2, where R is radius and ω is the angular velocity. For thin film deposition commercially produced (Sun Nanotech Co Ltd., China) CNTs powder were used. The diameter of multiwalled nanotubes (MWNTs) varied between 10-30 nm. Figure 1(a) shows the molecular structure of the orange dye (OD) and Figure 1 (b) shows schematic diagrams of the samples. The thickness of the films, the gap between electrodes and sizes of samples were in the range of 8-14 μm, 30-40 μm and 8 × 8 mm2, respectively. It was observed that the thickness of the films deposited at 1 g was usually larger than deposited at 70 g. It means the thickness of the films decreased with increase of acceleration. A similar behavior was observed in an earlier study as well[7]. The CNTs were in the form of suspension in the OD solution. The glass substrates with preliminary deposited copper films were placed inside a glass tube, which was mounted in the centrifugal machine. The internal diameter and length of the tubes used in the centrifugal process was 12 mm and 95 mm, respectively. The centrifuge rotation speed was set at 5000 rpm. For every experiment two symmetrically installed glass tubes filled with the solution of equal volume of 0.5 mL were used. In the centrifugal process the solution was evaporated at room temperature and atmospheric pressure without any additional heating.
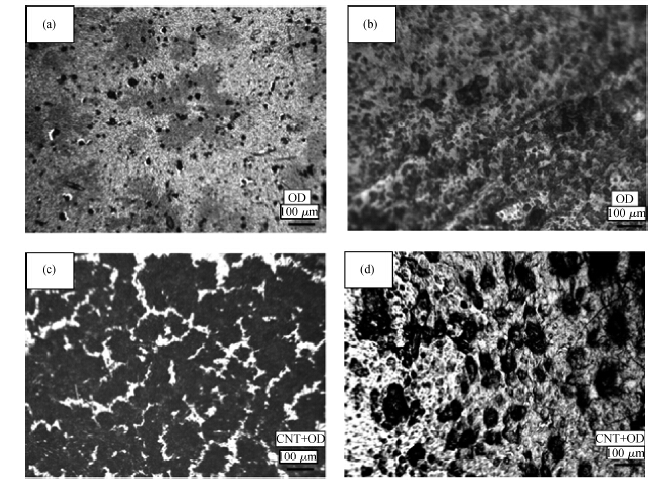
Figures 2(a) and 2(b) show optical microscope images of the orange dye films deposited at 1 g and 70 g from aqueous solution. Figures 2(c) and 2(d) show optical microscope images of the OD-CNT films deposited at 1 g and 70 g from aqueous solutions , respectively. It is seen that CNT and OD-CNT films deposited at 1 g are more uniform than those deposited at 70 g. For the measurements of impedance, phase angle, capacitance and dissipation of the samples, LCR meter MT 4090 was used at the applied frequency of 1 kHz and at a voltage level of 1 V, respectively. The relative humidity levels were measured by TECPEL 322 humidity meter. Experiments were conducted at room temperature conditions by use of a humidity controlled chamber for the humidity measurements.
3. Results and discussion
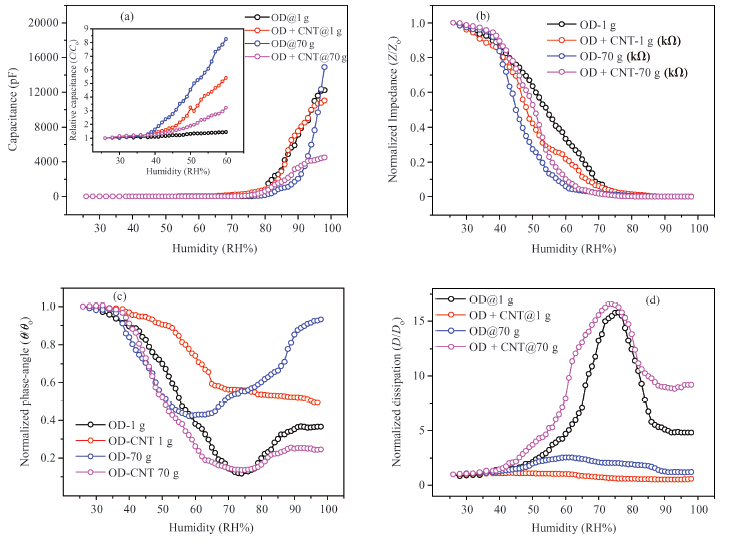
Figures 3(a) and 3(b) show capacitance-humidity and impedance-humidity relationships for the OD and OD-CNT composite film deposited at different gravity conditions, whereas the Figures 3(c) and 3(d) show their phase angle-humidity and dissipation-humidity relationships. In the case of OD films, it is seen that the capacitance increases 9488 and 18742 times at 1 g and 70 g whereas the impedance decrease 20855 and 17428 times, respectively. It means that the capacitance of the OD sample deposited at 70 g has shown higher sensitivity than of that deposited at 1 g. Dissipation and phase angle-humidity relationships show similarity with accordingly maximum and minimum at around humidity of 74 % (film deposited at 1 g) and 58 % (film deposited at 70 g). In the case of OD-CNT composite films. It is seen that impedances at 1 g and 70 g decrease with humidity 1081 and 6990 times, whereas capacitance increases 1814 and 2638 times. It means the deposited at 70 g OD-CNT sample impedance showed higher sensitivity than of the deposited at 1 g. Dissipation and phase angle-humidity relationships show different behavior: dissipation has a maximum of 42 % (film deposited at 1 g) and 74 % (film deposited at 70 g), phase angle increases with increase of humidity (film deposited at 1 g) or phase angle shows minimum (film deposited at 70 g).
The value of the capacitance depends on the polarizability of the material, which has several basic sources i.e. dipolar αdip, ionic αi and electronic αe polarizability[12]. In this case the dipolar (αdip) polarizability due to the presence of dipoles (H2O) absorbed by the OD seems to play a very important role. Electronic polarizability is universal and arises due to the relative displacement of the orbital electrons. OD may comprise charge-transfer complexes with water molecules; it can be assumed that ionic polarization takes place as well in this organic material. The increase in capacitance with increase in humidity can be explained in the following way. The dielectric permittivity of the OD increases due to adsorption of water molecules, a water molecule has higher dielectric permittivity value, by diffusion through the surface of the OD. The decrease in resistance and increase in capacitance, firstly, may be due to the presence of displacement current caused by water molecules, and secondly, may be due to possible doping of the OD by the water molecules and increase of the polarizability and concentration of charges related to the presence of the extra charge carriers. These mechanisms have been described in detail with respect to some solids[13].
The effect of humidity on the impedance and capacitance of the OD and OD-CNT composites can be estimated by the sensitivity (S) as[14]:
| S(Z)=ΔZ/ΔRH, | (1) |
| S(C)=ΔC/ΔRH, | (2) |
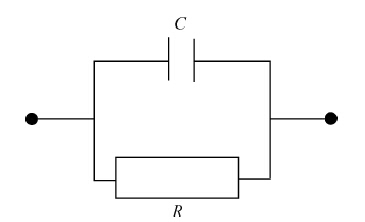
The impedance of the samples can be represented as parallel connection of resistance and capacitance (Figure 3). The physical reason for the effect of the humidity on the resistance and capacitance of the samples can be explained as an effect of adsorption and absorption of water molecules by the OD and OD-CNT composites. In the result of these processes obviously permittivity of the organic material increases that will bring an increase of capacitance. At the same time due to displacement currents related to movement of bound charges of water molecules the resistance of the samples may decrease. Finally the impedance of the samples decreases due to the increase of the capacitance and decrease of the resistance at the effect of humidity that is observed experimentally.
From an electronic point of view impedance (Z), resistance (R) and capacitance (C) have the following relationship[14]:
|
Z=R/(1+jωRC). |
(3) |
At the same time phase angle (θ) depends on conductance (G) and admittance (Y) ratio[15]:
| cosθ=G/Y=Z/R, | (4) |
The experimental results show that θ-(RH %) graphs are complicated; these graphs can be explained as a variation of contribution of resistance and capacitance into impedance under the effect of humidity (Figure 4). As is well known the dissipation (D) depends on capacitance and resistance and can be presented by the following equation[16]:
|
D=1/(2πfCR). |
(5) |
Under the increased humidity levels, the capacitance increases and resistance decreases whereas the dissipation-humidity relationship depends on whether the change of resistance is more than the change of the capacitance or vice versa, that is observed experimentally as shown in Figure 3.
Actually impedance sensitivity is higher at lower humidities, whereas the capacitance sensitivity is higher at higher humidities. It means practically it would be reasonable to measure humidity by these sensors at lower humidities in the mode of impedance measurement and at higher humidities in the mode of capacitance measurement. Moreover a humidity sensing unit can be comprised of two OD sensors, one of that fabricated at 1 g (for impedance measurement), another at 70 g (for capacitance measurement). It will allow maximum sensitivity to succeed at a wide range of humidity values. The effect of humidity on the impedance and capacitance of OD and OD-CNT composites potentially can be used in the fabrication of the humidity sensors that can be used in humidity meters for environmental monitoring and assessment. The detailed interpreptation of the results obtained by us will be given elsewhere. Centrifugation is used in different areas of technology. Carbon coating on graphite substrates using centrifugal deposition process was realized by Ahmadi et al.[17]. They also investigated the effect of centrifugal rotation speed and heat treatment on coating quality. At present, the investigations are happening not only in the area of high gravity processing of semiconductor materials but in the area of microgravity as well. In Reference [18] were described the properties of the tellurium crystals, Te-Se and Te80Si20 allowed to grow under microgravity level and up to 10 g. In particular, was observed the influence of gravity on distribution of defects in the crystals, on conductivity, and on Hall effect mobility as well.
4. Conclusion
Deposition of orange dye (OD) and OD-CNTs composites thin films at 1 g and 70 g showed that films deposited at 70 g showed more roughness or less uniformity with respect to films deposited at 1 g as was observed in the images obtained by optical microscopes. Impedance and capacitance sensitivities of the OD samples to humidity were higher than of OD-CNT samples. Capacitance sensitivity of the OD samples deposited at 70 g is higher than of the samples deposited at 1 g. The impedance of the OD-CNT sample deposited at 70 g showed higher sensitivity than of the one deposited at 1 g. The impedance greatly decreases at lower humidity, but capacitance increases at higher humidity. Practically it would be reasonable to measure humidity by these sensors at lower humidity in the mode of impedance measurement, and at higher humidity in the mode of capacitance measurement.





 DownLoad:
DownLoad:




 DownLoad:
DownLoad: